A spoonful of sugar is added to a hot cup of tea. All the sugar dissolves. How can the resulting solution be described?
A. Saturated and homogeneous
B. Saturated and heterogeneous
C. Unsaturated and homogeneous
D. Unsaturated and heterogeneous
Because more solute could be added and dissolve, the solution has not yet reached its limit and is considered unsaturated. Because all the solute dissolves, the particles in the mixture are evenly distributed as a homogenous mixture.
- A mixture is when elements and compounds are physically, but not chemically, combined.
- A homogeneous mixture is when substances mix evenly and it is impossible to see individual components. A heterogeneous mixture is when the substances mix unevenly and it is possible to see individual components.
- A solution is a type of homogeneous mixture that is formed when a solute dissolves in a solvent.
- The concentration of a solution is the amount of a substance in a given amount of solution. An unsaturated solution has the ability to dissolve more solute and a saturated solution has already reached the limit of solute it can dissolve.
Therefore, the Correct Answer is C.
More Questions on TEAS 7 Science
-
Q #1: In the following single-replacement reaction, ______ replaces ______. Cl2+2NaI→2NaCl+I2
A. sodium, iodine
B. chlorine, iodine
C. chlorine, sodium
D. sodium, chlorine
Answer Explanation
In this reaction, chlorine (Cl2) is an element in the reaction that replaces iodine in the compound sodium iodide (NaI). This allows chlorine to form a compound with sodium (NaCl) and leaves iodine (I2) as an element.
Synthesis reactions involve two or more reactants (A and B) combining to form one product (AB). In the example provided, hydrogen (H2) and oxygen (O2) begin as separate elements. At the end of the reaction, the hydrogen and oxygen atoms are bonded in a molecule of water (H2O).
Decomposition reactions have only one reactant (AB) that breaks apart into two or more products (A and B). In the example above, hydrogen peroxide (H2O2) breaks apart into two smaller molecules: water (H2O) and oxygen (O2).
Single-replacement reactions involve two reactants, one compound (AB) and one element (C). In this type of reaction, one element replaces another to form a new compound (AC), leaving one element by itself (B). In the example, zinc replaces hydrogen in hydrochloric acid (HCl). As a result, zinc forms a compound with chlorine, zinc chloride (ZnCl2), and hydrogen (H2) is left by itself.
Double-replacement reactions involve two reactants, both of which are compounds made of two components (AB and CD). In the example, silver nitrate, composed of silver (Ag1+) and nitrate (NO31-) ions, reacts with sodium chloride, composed of sodium (Na1+) and chloride (Cl1-) ions. The nitrate and chloride ions switch places to produce two compounds that are different from those in the reactants.
Combustion reactions occur when fuels burn, and they involve specific reactants and products, as seen in the examples below. Some form of fuel that contains carbon and hydrogen is required. Examples of such fuels are methane, propane in a gas grill, butane in a lighter, and octane in gasoline. Notice that these fuels all react with oxygen, which is necessary for anything to burn. In all combustion reactions, carbon dioxide, water, and energy are produced. When something burns, energy is released, which can be felt as heat and seen as light.
-
Q #2: _____ is dependent not only on the temperature, but also on the amount of substance available.
A. Condensation
B. Deposition
C. Evaporation
D. Melting
Answer Explanation
Unlike condensation, deposition, and melting, evaporation is dependent not only on the temperature, but also on the amount of a substance available.
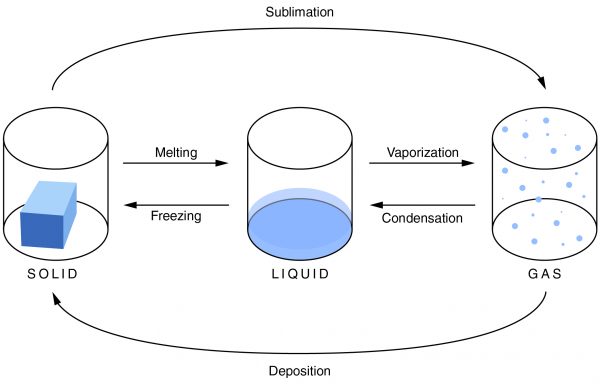
Condensation is the change of a gas or vapor to a liquid. A change in the pressure and the temperature of a substance causes this change. The condensation point is the same as the boiling point of a substance. It is most noticeable when there is a large temperature difference between an object and the atmosphere. Condensation is also the opposite of evaporation.
Evaporation is the change of a liquid to a gas on the surface of a substance. This is not to be confused with boiling, which is a phase transition of an entire substance from a liquid to a gas. The evaporation point is the same as the freezing point of a substance. As the temperature increases, the rate of evaporation also increases. Evaporation depends not only on the temperature, but also on the amount of substance available.
Freezing is the change of a liquid to a solid. It occurs when the temperature drops below the freezing point. The amount of heat that has been removed from the substance allows the particles of the substance to draw closer together, and the material changes from a liquid to a solid. It is the opposite of melting.
Melting is the change of a solid into a liquid. For melting to occur, enough heat must be added to the substance. When this is done, the molecules move around more, and the particles are unable to hold together as tightly as they can in a solid. They break apart, and the solid becomes a liquid.
Sublimation is a solid changing into a gas. As a material sublimates, it does not pass through the liquid state. An example of sublimation is carbon dioxide, a gas, changing into dry ice, a solid. It is the reverse of deposition.
Deposition is a gas changing into a solid without going through the liquid phase. It is an uncommon phase change. An example is when it is extremely cold outside and the cold air comes in contact with a window. Ice will form on the window without going through the liquid state.
-
Q #3: Which of the following types of tissues include cells of the immune system and of the blood?
A. Connective
B. Epithelial
C. Muscle
D. Neural
Answer Explanation
A tissue is a group of cells with similar structure and function and similar extracellular substances located between the cells. The table below describes the four primary tissues found in the human body.
body.
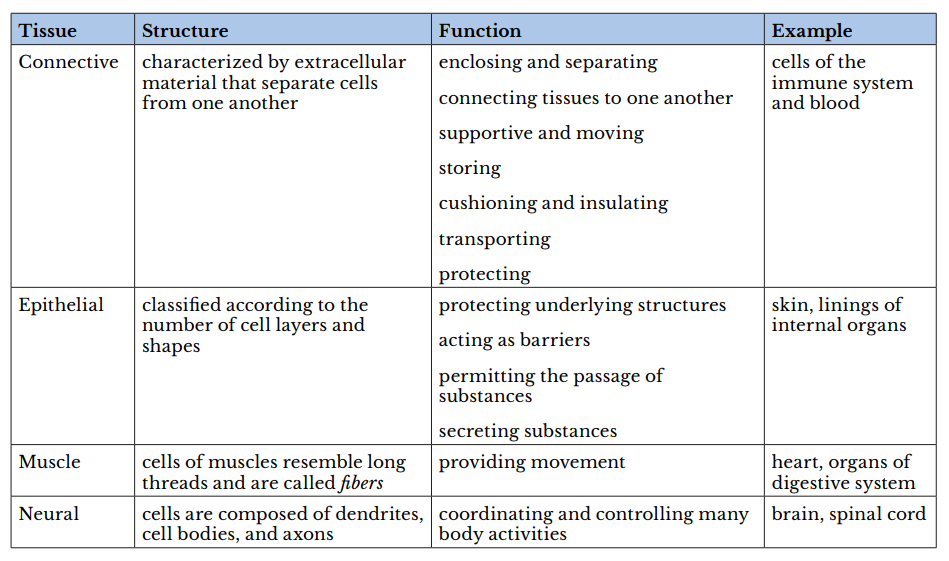
-
Q #4: While hiking, a person is startled after encountering a bear. Her palms get sweaty and her heart starts racing. Which part of her nervous system was directly stimulated?
A. Central
B. Parasympathetic
C. Somatic
D. Sympathetic
Answer Explanation
The autonomic nervous system is responsible for activities that are nonvoluntary and under unconscious control. This system controls glands and the smooth muscles of internal organs, heart rate, breathing, and digestion. The autonomic nervous system is further divided into the following:
- Sympathetic nervous system: The sympathetic nervous system focuses on emergency situations by preparing the body for fight or flight. (Sympathetic = Stress)
- Parasympathetic nervous system: The parasympathetic nervous system controls involuntary processes unrelated to emergencies. This system deals with “rest or digest” activities. (Parasympathetic = Peace)
The somatic nervous system primarily controls voluntary activities such as walking and riding a bicycle. Thus, this system sends information to the CNS and motor nerve fibers that are attached to skeletal muscle.
-
Q #5: An atom has 28 protons, 32 neutrons, and 28 electrons. What is the name of this isotope?
A. Nickel-32
B. Nickel-60
C. Germanium-56
D. Germanium-60
Answer Explanation
The number of protons, 28, gives the atomic number, which identifies this atom as nickel. The mass is the number after the dash in the isotope name, which is determined by adding the numbers of protons and neutrons (28 + 32 = 60).
-
Q #6: The sequence of amino acids in a gene determines
A. the primary structure of a codon
B. the primary structure of a protein
C. the primary structure of a nucleotide
D. the primary structure of a nucleic acid.
Answer Explanation
The sequence of amino acids in a gene determines the primary structure of a protein. The components necessary for translation are located in the cytoplasm. Translation is the making of proteins by mRNA binding to a ribosome with the start codon that initiates the production of amino acids. A peptide bond forms and connects the amino acids together. The sequence of amino acids determines the protein’s structure, which determines its function.
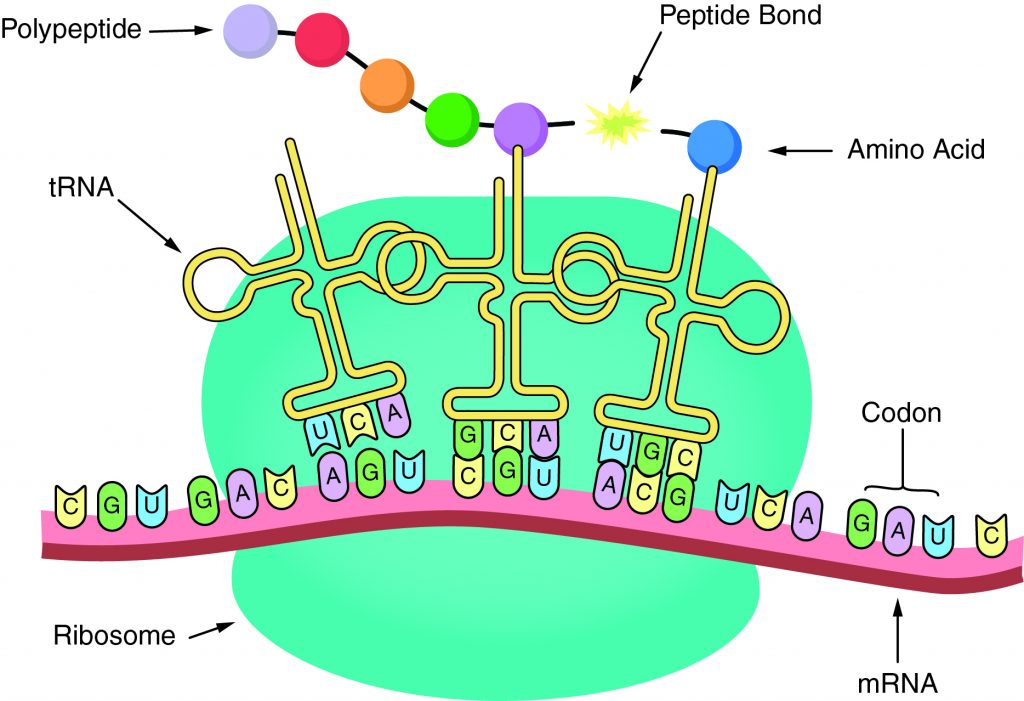
-
Q #7: Which choice best describes homeostasis?
A. A functional system of the body
B. Blood flow to every cell in the body
C. A relatively constant environment within the body
D. Neural pathways that have integrated into the body
Answer Explanation
Homeostasis is the existence and maintenance of a relatively constant environment within the body. Each cell of the body is surrounded by a small amount of fluid, and the normal functions of each cell depend on the maintenance of its fluid environment within a narrow range of conditions, including temperature, volume, and chemical content. These conditions are known as variables. For example, body temperature is a variable that can increase in a hot environment or decrease in a cold environment.
There are two types of feedback mechanisms in the human body: negative and positive.
- Negative Feedback: Most systems of the body are regulated by negative feedback mechanisms, which maintain homeostasis. Negative means that any deviation from the set point is made smaller or is resisted. The maintenance of normal blood pressure is a negative-feedback mechanism. Normal blood pressure is important because it is responsible for moving blood from the heart to tissues.
- Positive Feedback: Positive-feedback mechanisms are not homeostatic and are rare in healthy individuals. Positive means that when a deviation from a normal value occurs, the response of the system is to make the deviation even greater. Positive feedback therefore usually creates a cycle leading away from homeostasis and, in some cases, results in death. Inadequate delivery of blood to cardiac muscle is an example of positive feedback.
-
Q #8: An intracellular chemical signal can be produced in the cell membrane. Once it is produced, where does it go?
A. To a different cell
B. To another part of the same cell
C. To a region right outside the cell
D. To an area with a high ion concentration
Answer Explanation
There are two major types of receptor molecules that respond to an intercellular chemical signal:
- Intracellular receptors: These receptors are located in either the cytoplasm or the nucleus of the cell. Signals diffuse across the cell membrane and bind to the receptor sites on intracellular receptors, of the same cell.
- Membrane-bound receptors: These receptors extend across the cell membrane, with their receptor sites on the outer surface of the cell membrane. They respond to intercellular chemical signals that are large, water-soluble molecules that do not diffuse across the cell membrane.
-
Q #9: Fertilization (the fusing of one sperm and an ovum) results in a(n) _____.
A. embryo
B. fetus
C. infant
D. zygote
Answer Explanation
Human intercourse consists of the male introducing sperm into the female’s reproductive system. Sperm may then pass through the female’s reproductive system to the Fallopian tubes where one sperm fertilizes an ovum, creating a zygote. The zygote passes out of the Fallopian tube and implants into the uterine wall to begin gestation. Over nine months, the zygote develops and grows into an embryo and then a fetus. An infant is the baby that is born.
-
Q #10: What solution has a pH of 7?
A. Aniline
B. Pyridine
C. Pure water
D. Sodium hydroxide
Answer Explanation
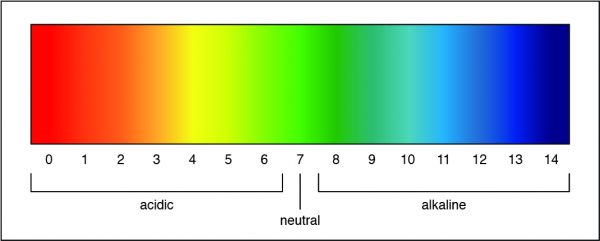
A pH of 7 is a neutral solution, which is how pure water is classified. Researchers can determine the strength of an acid or a base by measuring the pH of a solution. The pH value describes how acidic or basic a solution is. On pH scale, shown below, if the number is less than 7 the solution is acidic. A pH greater than 7 means the solution is basic. When the pH is exactly 7, the solution is neutral.
Free Access on TEAS 7 Exams and Study Notes
- Access to all TEAS 7 Exams
- Performance Tracking and Analysis
- Well Documented and Explained Questions and Answers
- 2000+ Questions and Correct Answers: Answers Well Explained
- Libary of Detailed StudyNotes
- Topical Questions and Answers on Examinable topics
TEAS 7 Exams (Q&A)
TEAS 7 Study Notes
TEAS 7 Topical Tests

TEAS 7 Study Guides
Quick Links
Refer a Friend
Refer a friend and claim free unlimited access

© 2024 ExamGates Made with by ExamGates
