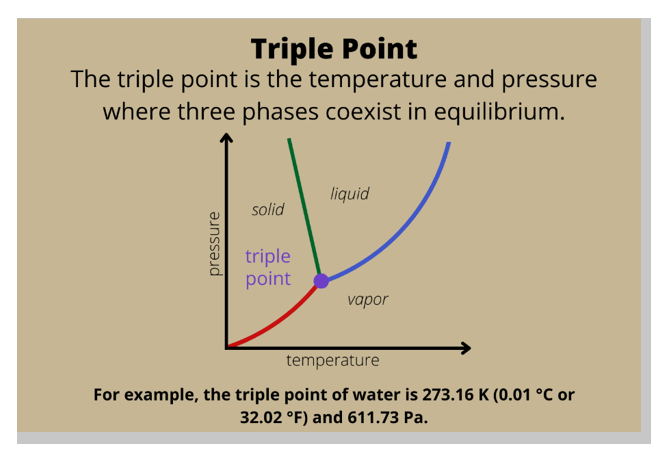
In a phase diagram, which of the following is the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously?
A. Triple point
B. Critical temperature
C. Critical point
D. Absolute zero
Triple point.
In a phase diagram, the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously is the triple point.
The triple point is a unique point on a phase diagram where the three states of matter (solid, liquid, and gas) can coexist in equilibrium.
At the triple point, the temperature and pressure of the substance are fixed.
Option B, critical temperature, is the temperature at which a gas cannot be liquefied, regardless of the pressure applied.
It is a characteristic property of a substance and is typically higher than the boiling point of the liquid at standard pressure.
Option C, critical point, is the point on a phase diagram where the liquid and gas phases of a substance become indistinguishable.
At the critical point, the distinction between the liquid and gas phases disappears, and the substance becomes a supercritical fluid.
Option D, absolute zero, is the theoretical temperature at which all matter has zero thermal energy.
At absolute zero, all substances are in their solid state, but it is not relevant to a phase diagram, as it is a temperature where no transitions between states occur.
In summary, the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously in a phase diagram is the triple point, whereas the other options provided are not relevant or are characteristic properties of substances in different contexts.
Therefore, the Correct Answer is A.
More Questions on TEAS 7 Science
-
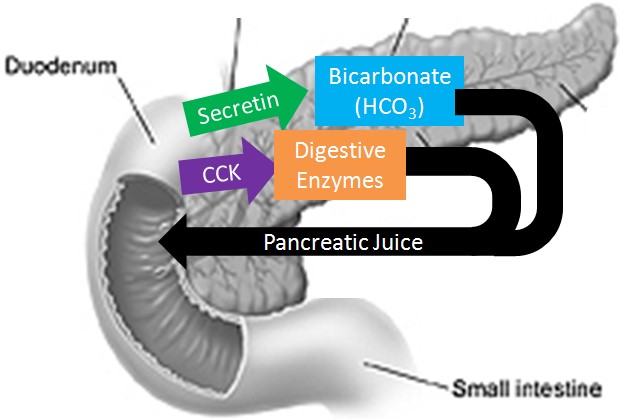
Q #1: Which of the following indicates the function of sodium bicarbonate secreted by the pancreas?
A. Sodium bicarbonate is a protease that digests carbohydrates.
B. Sodium bicarbonate stimulates the pyloric sphincter.
C. Sodium bicarbonate inhibits peristalsis.
D. Sodium bicarbonate neutralizes the acidity of chyme.
Answer Explanation
Sodium bicarbonate neutralizes the acidity of chyme.
The pancreas secretes large amounts of sodium bicarbonate, which protects the duodenum by neutralizing the acid that comes from the stomach.
This compound helps neutralize stomach acid generated during the digestive process.
Choice A is incorrect because sodium bicarbonate is not a protease that digests carbohydrates.
Proteases are enzymes that break down proteins, while sodium bicarbonate is a chemical compound that helps neutralize stomach acid.
Choice B is incorrect because sodium bicarbonate does not stimulate the pyloric sphincter.
The pyloric sphincter is a ring of smooth muscle that separates the stomach from the duodenum and regulates the passage of partially digested food (chyme) into the small intestine.
Choice C is incorrect because sodium bicarbonate does not inhibit peristalsis.
Peristalsis is a series of wave-like muscle contractions that move food through the digestive tract.
-
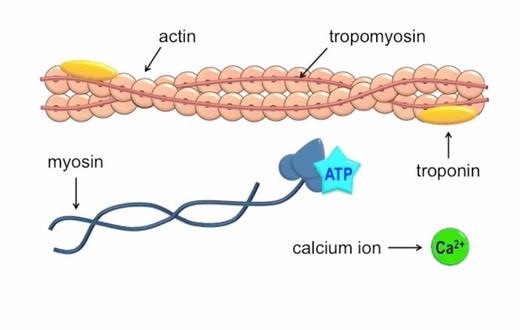
Q #2: Which of the following ions binds to the troponin complex, initiating contraction of a muscle?
A. Potassium.
B. Calcium.
C. Phosphorus.
D. Sodium
Answer Explanation
Calcium ions play a crucial role in initiating muscle contraction.
When a muscle cell is stimulated to contract by an action potential, calcium channels open in the sarcoplasmic membrane and release calcium into the sarcoplasm.
Some of this calcium attaches to troponin, which causes it to change shape.
This shape change exposes binding sites for myosin on the actin filaments.
Myosin’s binding to actin causes crossbridge formation, and contraction of the muscle begins.
The other ions mentioned in the question do not have this specific role in muscle contraction.
Potassium ions are important for maintaining the resting membrane potential of cells, but they do not bind to the troponin complex.
Phosphorus ions are important for energy metabolism, but they do not bind to the troponin complex.
Sodium ions are important for generating action potentials, but they do not bind to the troponin complex.
-
Q #3: Which of the following is a group that can be measured against the experimental group?
A. Responding
B. Manipulated
C. Control
D. Variable
Answer Explanation
Control.
A control group is a group in an experiment that does not receive the treatment or manipulation being tested and is used as a benchmark to measure how the other tested subjects do.
The control group is used to minimize the effects of all variables except the independent variable.
This allows researchers to determine if changes in the dependent variable are due to the manipulation of the independent variable or if they are due to some other factor.
Choice A.
Responding is not the correct answer because it refers to the dependent variable, which is the variable that is being measured in an experiment.
Choice B.
Manipulated is not the correct answer because it refers to the independent variable, which is the variable that is being manipulated in an experiment.
Choice D.
Variable is not the correct answer because it refers to any factor that can change in an experiment and can include both independent and dependent variables.
-
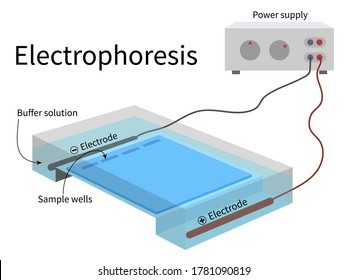
Q #4: To separate genomic DNA fragments by size, which of these laboratory methods is most useful?
A. Titration
B. Electrophoresis
C. Filtration
D. Spectrophotometry
Answer Explanation
Electrophoresis is the most useful laboratory method for separating genomic DNA fragments by size.
Electrophoresis is a technique that uses an electric field to separate charged molecules, such as DNA fragments, based on their size and charge.
Choice A is not correct because titration is a laboratory method used to determine the concentration of a solution.
Choice C is not correct because filtration is a laboratory method used to separate solids from liquids.
Choice D is not correct because spectrophotometry is a laboratory method used to measure the absorbance of light by a solution.
-
Q #5: Which of the following best describes the result of using a catalyst in a chemical reaction?
A. The reaction is completed in a shorter amount of time
B. A more desirable product is often formed
C. A greater amount of heat energy is released by the reaction
D. The yield of product is increased
Answer Explanation
A catalyst is a substance that increases the rate of a chemical reaction without being consumed by the reaction.
As a result, the reaction is completed in a shorter amount of time.
Choice B is not correct because using a catalyst does not necessarily result in the formation of a more desirable product.
Choice C is not correct because using a catalyst does not necessarily result in the release of a greater amount of heat energy by the reaction.
Choice D is not correct because using a catalyst does not necessarily increase the yield of product.
-
Q #6: Nitrogen gas is an extremely stable molecule because of which of the following?
A. Ionic bonds
B. Hydrogen bonds
C. Resonance bonds
D. Triple covalent bonds
Answer Explanation
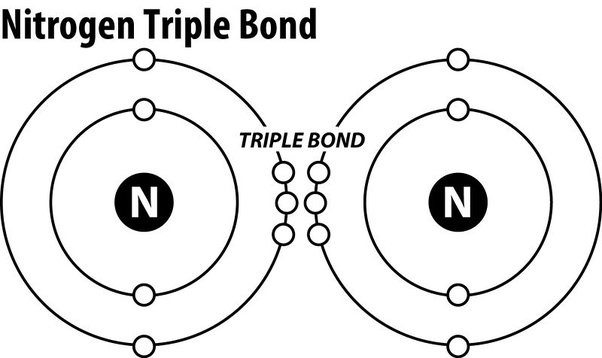
Triple covalent bonds.
Nitrogen gas (N2) is an extremely stable molecule because it consists of two nitrogen atoms bonded together by a triple covalent bond.
A covalent bond is a type of chemical bond where atoms share electrons to form a molecule.
In a triple covalent bond, three pairs of electrons are shared between the two atoms, resulting in a very strong bond that makes the molecule extremely stable.
Choice A.
Ionic bonds is not correct because ionic bonds involve the transfer of electrons from one atom to another to form ions, which are then attracted to each other due to their opposite charges.
Nitrogen gas does not contain ions and is not held together by ionic bonds.
Choice B.
Hydrogen bonds is not correct because hydrogen bonds are weak electrostatic attractions between molecules that contain hydrogen atoms bonded to highly electronegative atoms such as oxygen or nitrogen.
Nitrogen gas does not contain hydrogen atoms and is not held together by hydrogen bonds.
Choice C.
Resonance bonds is not correct because resonance refers to the delocalization of electrons in a molecule where multiple Lewis structures can be drawn to represent the molecule.
Nitrogen gas has a single Lewis structure and does not exhibit resonance.
-
Q #7: Which of the following summarizes a change that takes place as a solid turns to a liquid?
A. Particles become less ordered.
B. Particles have a decrease in mobility.
C. Particles move closer together
D. Intermolecular forces between particles become stronger.
Answer Explanation
As a solid turns to a liquid, the particles become less ordered and more free to move around.
Choice B is not correct because particles have an increase in mobility as a solid turns to a liquid.
Choice C is not correct because particles move further apart as a solid turns to a liquid.
Choice D is not correct because intermolecular forces between particles become weaker as a solid turns to a liquid.
-
Q #8: The measurement indicated by the line across the center of the cell is best referred to as which of the following?
A. Area.
B. Diameter.
C. Volume.
D. Radius.
Answer Explanation
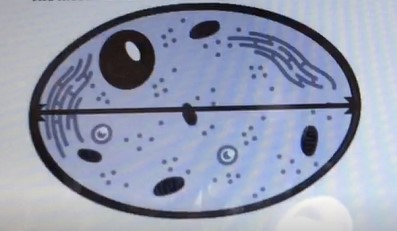
Diameter.
The diameter is the measurement of a straight line passing through the center of a circle and connecting two points on its circumference.
In this case, the line across the center of the cell represents the diameter of the cell.
Choice A, Area, is not the correct answer because area refers to the amount of space inside a two-dimensional shape.
Choice C, Volume, is not the correct answer because volume refers to the amount of space occupied by a three-dimensional object.
Choice D, Radius, is not the correct answer because radius refers to the distance from the center of a circle to its circumference and is half the length of the diameter.
-
Q #9: Which of the following is the process in which an ovarian follicle matures and releases a reproductive egg?
A. Menstruation
B. Fertilization
C. Ovulation
D. Oogenesis
Answer Explanation
Ovulation is the process in which an ovarian follicle matures and releases a reproductive egg.
During ovulation, a mature egg is released from the female ovary, enabling it to be fertilized by male sperm cells 1.
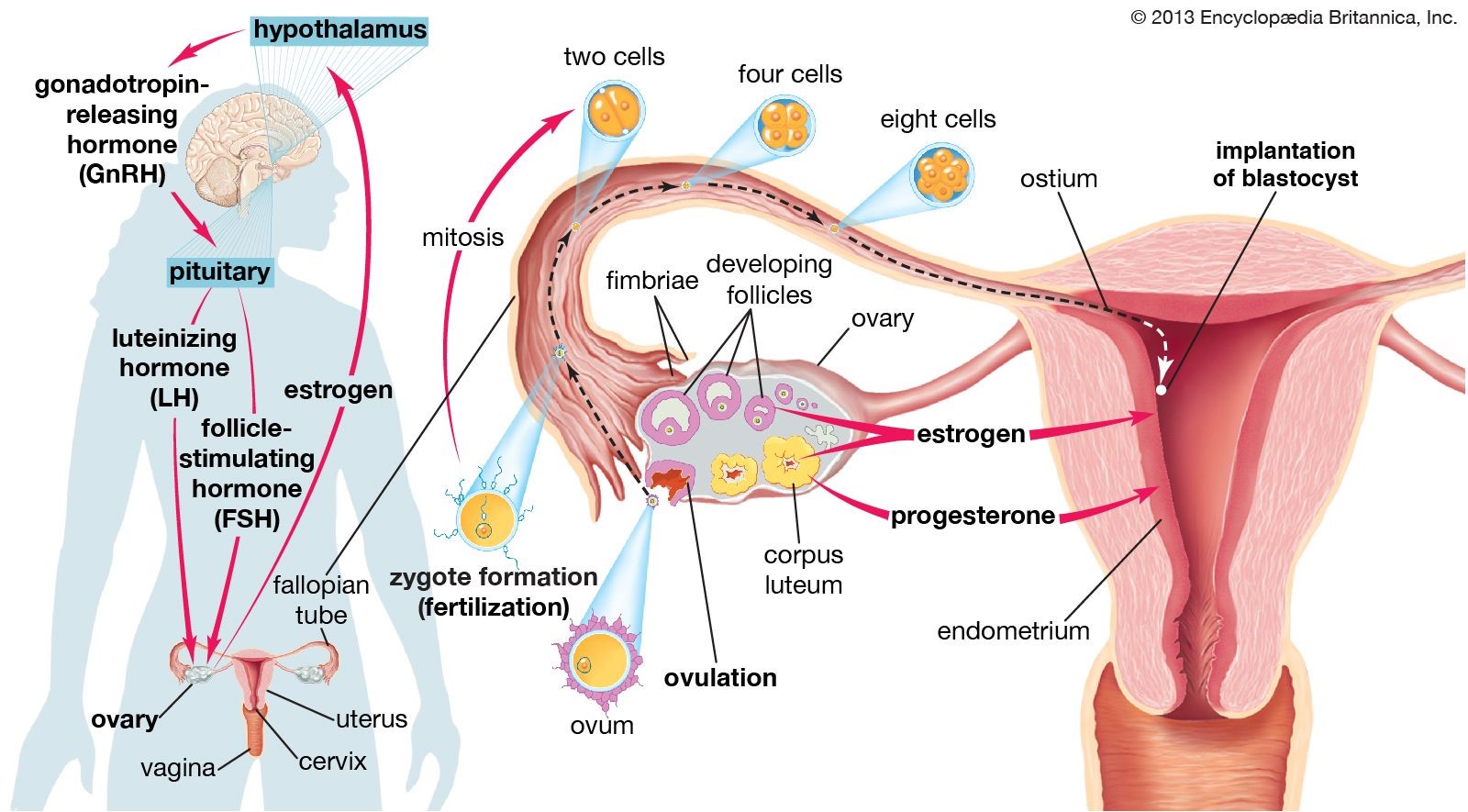
Choice A is incorrect because menstruation is the process of shedding the uterine lining, which occurs when an egg is not fertilized.
Choice B is incorrect because fertilization is the process of a sperm cell joining with an egg cell to form a zygote.
Choice D is incorrect because oogenesis is the process of forming female gametes (eggs) in the ovaries.
-
Q #10: Which of the following results in osteoporosis?
A. An increase in osteocyte activity while osteoclast activity continues at expected levels.
B. A decline in osteoclast activity while osteoblast activity continues at expected levels.
C. An increase in osteocyte activity while osteoblast activity reduces.
D. A decline in osteoblast activity while osteoclast activity continues at expected levels.
Answer Explanation
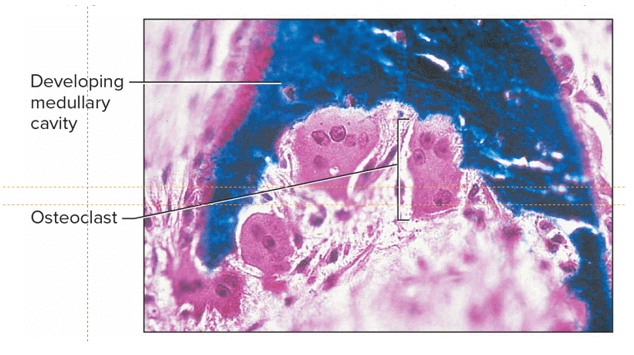
A decline in osteoblast activity while osteoclast activity continues at expected levels results in osteoporosis.
Osteoporosis is caused by an imbalance between the functioning of osteoclast and osteoblast cells.
Osteoblasts are responsible for forming new bone, while osteoclasts break down old bone.
If osteoblast activity declines while osteoclast activity continues at expected levels, this means that more bone is being broken down than is being formed, leading to a loss of bone density and an increased risk of osteoporosis.
Choice A is incorrect because an increase in osteocyte activity would not result in osteoporosis.
Osteocytes are mature bone cells that maintain the mineral concentration of the bone matrix.
Choice B is incorrect because a decline in osteoclast activity would not result in osteoporosis.
Osteoclasts break down old bone, so a decline in their activity would mean that less bone is being broken down.
Choice C is incorrect because an increase in osteocyte activity would not result in osteoporosis.
As mentioned earlier, osteocytes are mature bone cells that maintain the mineral concentration of the bone matrix.
Free Access on TEAS 7 Exams and Study Notes
- Access to all TEAS 7 Exams
- Performance Tracking and Analysis
- Well Documented and Explained Questions and Answers
- 2000+ Questions and Correct Answers: Answers Well Explained
- Libary of Detailed StudyNotes
- Topical Questions and Answers on Examinable topics
TEAS 7 Exams (Q&A)
TEAS 7 Study Notes
TEAS 7 Topical Tests

TEAS 7 Study Guides
Quick Links
Refer a Friend
Refer a friend and claim free unlimited access

© 2024 ExamGates Made with by ExamGates
