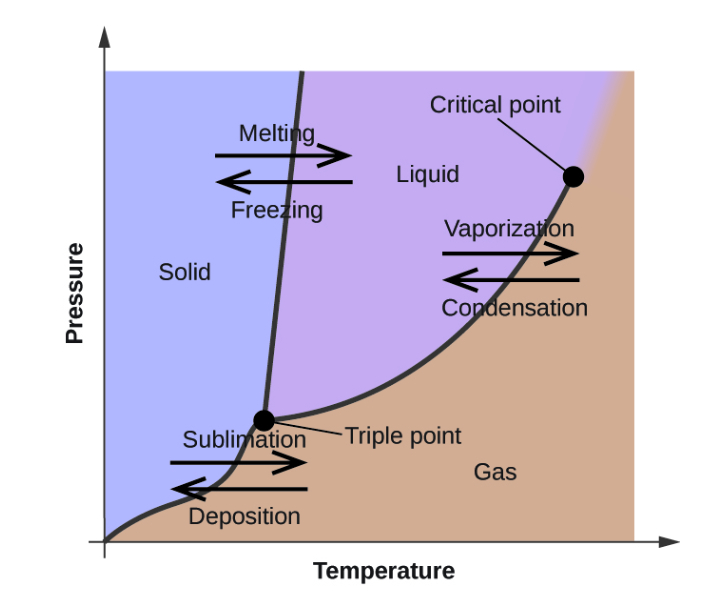
When the temperature of a liquid is increased a little, what happens to its particles?
A. They move so far apart that the liquid becomes a gas.
B. They start vibrating more.
C. They stop vibrating.
D. They come closer together.
As the temperature increase, the liquid turns into a gas. This process is known as vaporization, and in gaseous state the particles become far apart and move in random motion. The change is visually presented below
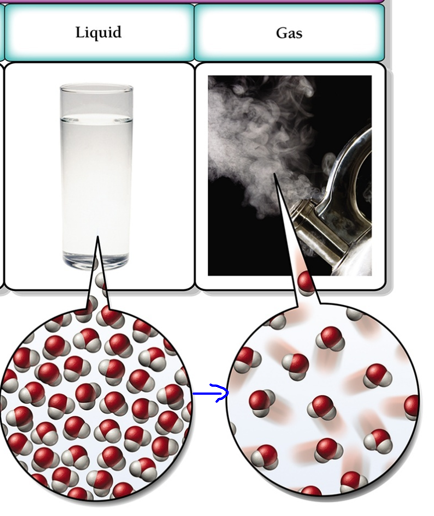
Therefore, the Correct Answer is A.