Which classification of the reaction shown below:
A. combination
B. combustion
C. single replacement
D. double replacement
Looking at the above chemical reaction, Al replaces H2 from sulfuric acid. This reaction is of single displacement type.
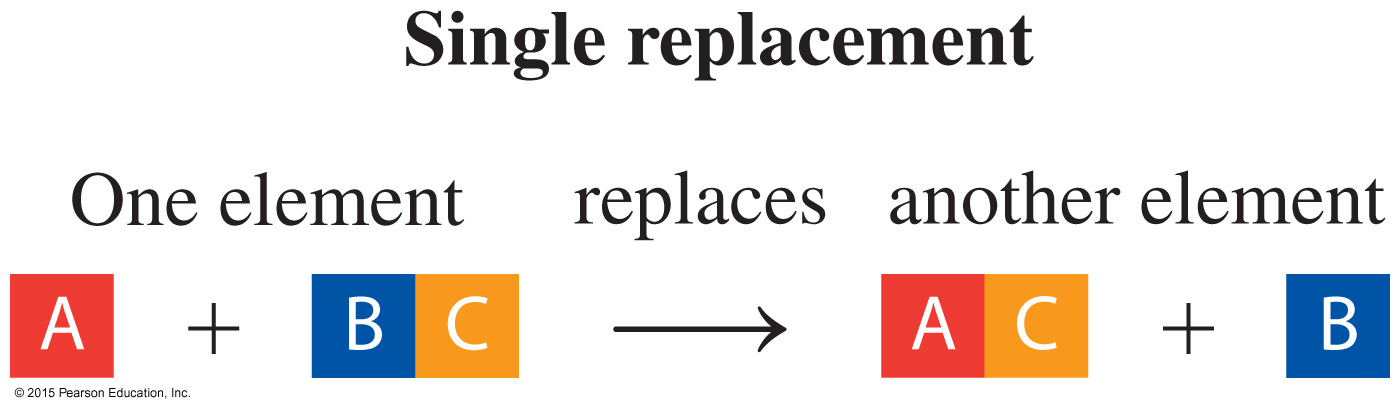
Therefore, the Correct Answer is C.


