Which is classified as a type of acid-base reaction that produces a salt?
A. Combination
B. Decomposition
C. Hydrolysis
D. Neutralization
A neutralization reaction is a type of acid-base reaction where an acid and base react to form a salt and water.
In an aqueous solution, a base increases the hydroxide concentration (OH–), while an acid increases the hydrogen ion (H+) concentration. Sometimes, neutralization reactions also occur. This type of reaction happens when an acid and a base react with each other to form water and salt. Salt is typically defined as an ionic compound that includes any cation except H+ and any anion except OH–. Consider the following example of a neutralization reaction between hydrobromic acid (HBr) and potassium hydroxide (KOH).
HBr+KOH→KBr+H2O
Not all neutralization reactions proceed in the manner where all reactants are in the aqueous phase. In some chemical reactions, one reactant may be a solid. The neutralization reaction can still proceed to completion.
Therefore, the Correct Answer is D.
More Questions on TEAS 7 Science
-
Q #1: As soon as an invader, known as a(n) _____, enters the body, the body begins to fight.
A. antibody
B. pathogen
C. trigger
D. vaccination
Answer Explanation
Pathogen is an infectious foreign body that enters the body and causes disease or illness to the person. There are five types of pathogens: viruses, bacteria, fungi, protozoa, and worms. Pathogens have antigen proteins found on their surface and are unique to each pathogen.
Antibody is a protein produced by the body’s immune system when it detects harmful substances (antigens). There are many different antibodies found in the body. Each one is unique and protects the body against the specific antigen that it detects at any given time. If there are no antibodies for a specific antigen, the more likely you are to develop an illness.
Vaccinations are the introduction of a dead or disabled pathogen or of a harmless microbe with the protein of a pathogen on its surface into the body. Often administered through needle injection, to stimulate the immune system to produce immunity to a specific disease Immunity protects the body from a disease when exposed to it.
There are four types of immunity: natural/passive, natural/active, artificial/passive, and artificial/ active.
- Natural/passive – Babies receive immunities from breastmilk.
- Natural/active – The body produces antibodies to combat an illness when a person becomes sick.
- Artificial/passive – This immunity is temporary and requires doses of serum to maintain the immunity.
- Artificial/active – A vaccination provides artificial/active immunity.
-
Q #2: Which of the following is a component of a chromosome?
A. Centromere
B. Gamete
C. Homologue
D. Ribose
Answer Explanation
The protein disc that holds two sister chromatids together is what collectively makes a chromosome. A gene is a segment of DNA, deoxyribonucleic acid, which transmits information from parent to offspring. A single molecule of DNA has thousands of genes. A chromosome is a rod-shaped structure that forms when a single DNA molecule and its associated proteins coil tightly before cell division.
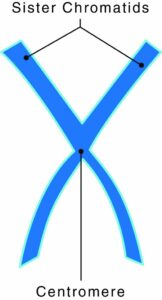
Chromosomes have two components:
- Chromatids: two copies of each chromosome
- Centromeres: protein discs that attach the chromatids together
Human cells have 23 sets of different chromosomes. The two copies of each chromosome are called homologous chromosomes, or homologues. An offspring receives one homologue from each parent. When a cell contains two homologues of each chromosome, it is termed diploid (2n). A haploid (n) cell contains only one homologue of each chromosome. The only haploid cells humans have are the sperm and eggs cells known as gametes.
-
Q #3: An intracellular chemical signal can be produced in the cell membrane. Once it is produced, where does it go?
A. To a different cell
B. To another part of the same cell
C. To a region right outside the cell
D. To an area with a high ion concentration
Answer Explanation
There are two major types of receptor molecules that respond to an intercellular chemical signal:
- Intracellular receptors: These receptors are located in either the cytoplasm or the nucleus of the cell. Signals diffuse across the cell membrane and bind to the receptor sites on intracellular receptors, of the same cell.
- Membrane-bound receptors: These receptors extend across the cell membrane, with their receptor sites on the outer surface of the cell membrane. They respond to intercellular chemical signals that are large, water-soluble molecules that do not diffuse across the cell membrane.
-
Q #4: What type of reaction is described by the following equation? ZnBr2(aq) + 2KOH(aq) → Zn(OH)2(s) + 2KBr(aq)
A. Synthesis
B. Decomposition
C. Single-Replacement
D. Double-Replacement
Answer Explanation
In this reaction, two elements are trading places hence double-replacement. In the reactants, zinc and bromide ions are together, and potassium and hydroxide ions are together. In the products, zinc and hydroxide ions are together, and potassium and bromide ions are together.
-
Q #5: In which state of matter do the particles of iron have the lowest amount of cohesion?
A. Solid iron particles have the lowest amount of cohesion
B. Liquid iron particles have the lowest amount of cohesion
C. Gaseous iron particles have the lowest amount of cohesion
D. The particles have the same amount of cohesion in all states of matter.
Answer Explanation
The particles in a sample of gas are farther apart than in solids or liquids and therefore have the lowest amount of cohesion.
- Cohesion is the tendency of particles of the same kind to stick to each other.
- A solid has the lowest amount of energy because its particles are packed close together. Liquids have more energy than a solid, and gases have more energy than solids or liquids because the cohesive forces are very weak.
-
Q #6: The sequence of amino acids in a gene determines
A. the primary structure of a codon
B. the primary structure of a protein
C. the primary structure of a nucleotide
D. the primary structure of a nucleic acid.
Answer Explanation
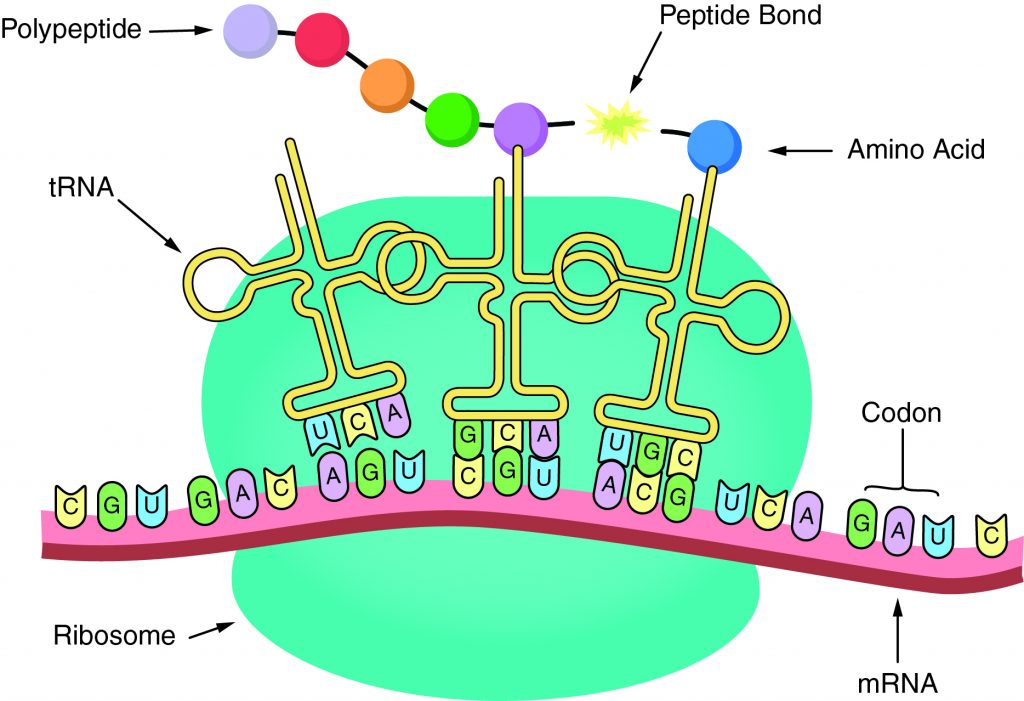
The sequence of amino acids in a gene determines the primary structure of a protein. The components necessary for translation are located in the cytoplasm. Translation is the making of proteins by mRNA binding to a ribosome with the start codon that initiates the production of amino acids. A peptide bond forms and connects the amino acids together. The sequence of amino acids determines the protein’s structure, which determines its function.
-
Q #7: Mendel discovered the pattern associated with _____after developing a series of rules in genetics.
A. epigenetics
B. heredity
C. heterogeneity
D. taxonomy
Answer Explanation
Mendel was accurately able to predict the patterns of heredity by studying rules related to genetics. These rules helped shape his theory of heredity. Heredity is the characteristics offspring inherit from their parents.
From experiments with garden peas, Mendel developed a simple set of rules that accurately predicted patterns of heredity. He discovered that plants either self-pollinate or cross-pollinate, when the pollen from one plant fertilizes the pistil of another plant. He also discovered that traits are either dominant or recessive. Dominant traits are expressed, and recessive traits are hidden.
Mendel’s Theory of Heredity
To explain his results, Mendel proposed a theory that has become the foundation of the science of genetics. The theory has five elements:
- Parents do not transmit traits directly to their offspring. Rather, they pass on units of information called genes.
- For each trait, an individual has two factors: one from each parent. If the two factors have the same information, the individual is homozygous for that trait. If the two factors are different, the individual is heterozygous for that trait. Each copy of a factor, or gene, is called an allele.
- The alleles determine the physical appearance, or phenotype. The set of alleles an individual has is its genotype.
- An individual receives one allele from each parent.
- The presence of an allele does not guarantee that the trait will be expressed.
-
Q #8: Which is true regarding the Urinary system?
A. Kidneys makes urine, Kidney help regulate water balance.
B. As a person ages, kidney tissue and filtration capacity increase, Regulates levels of electrolytes such as sodium and potassium.
C. Eliminates metabolic wastes., Kidneys makes urine., Kidney help regulate water balance.
D. Kidney help regulate water balance, Regulates levels of electrolytes such as sodium and potassium, Eliminates metabolic wastes
Answer Explanation
Kidneys makes urine is incorrect. Kidneys do not make urine. They help regulate water balance, regulate levels of electrolytes such as sodium and potassium, and eliminate metabolic wastes. Urine is a byproduct of these functions.
As a person ages, kidney tissue and filtration capacity increase is incorrect. As a person ages, the kidneys and bladder change. This can affect functions such as bladder control and how well the kidneys filter blood. Kidney changes range from a decrease in kidney tissue to decreased filtration capacity.
Kidneys help regulate water balance is correct. Kidneys help regulate water balance, regulate levels of electrolytes such as sodium and potassium, and eliminate metabolic wastes. Urine is a byproduct of these functions.
Regulates levels of electrolytes such as sodium and potassium is correct. There must be a continual balance of water and salt in the blood. The urinary system, specifically the kidneys, help maintain this balance. It also balances levels of metabolites or electrolytes such as sodium, potassium, and calcium.
Eliminates metabolic wastes is correct. Urea, creatinine, uric acid, and ammonium are the primary types of nitrogenous wastes excreted from the body. The urinary system also detects and excretes excess water from the blood and out of the body.
-
Q #9: In which state of matter are the intermolecular forces between particles in a substance the strongest?
A. Gas
B. Liquid
C. Plasma
D. Solid
Answer Explanation
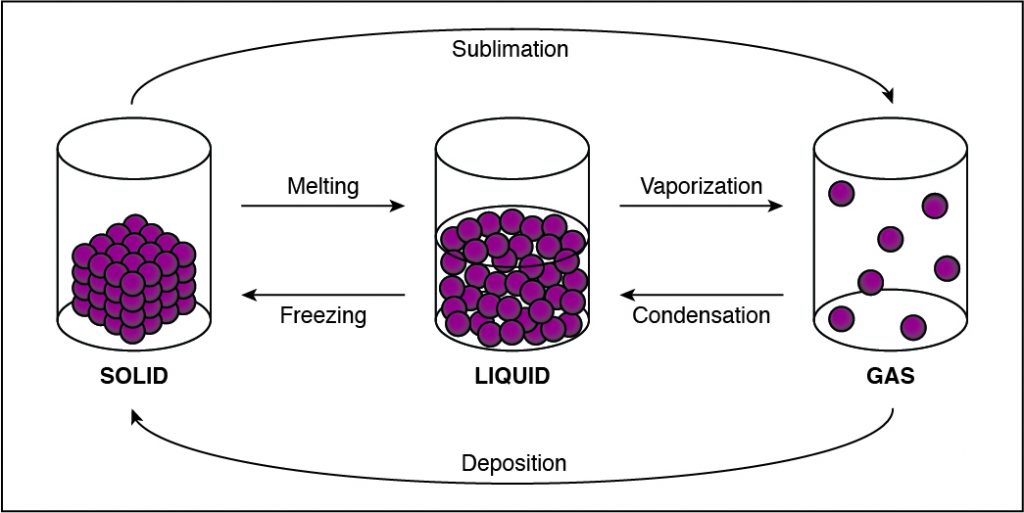
In solids, particles are usually closer together than in other states of matter because of the strong cohesive forces between the particles.
- Solids, liquids, gases, and plasmas differ from one another in the amount of energy that the particles have and the strength of the cohesive forces that hold the particles together.
- Cohesion is the tendency of particles of the same kind to stick to each other.
- A solid has the lowest amount of energy because its particles are packed close together. Liquids have more energy than a solid, and gases have more energy than solids or liquids because the cohesive forces are very weak.
-
Q #10: A person is diagnosed as having acidosis, a condition in which the blood pH is below 7.45. What does the doctor most likely conclude?
A. Too much carbon dioxide is found in the blood.
B. Highly oxygenated blood circulates through the body
C. A blockage prevents blood from leaving the pulmonary artery
D. The nasal cavity has a difficult time clearing particles from the air.
Answer Explanation
Acidosis is when the body fluids contain too much acid, or low pH. The kidneys and lungs are unable to keep the body’s pH in balance. Acidosis is the result when there is too much loss of bicarbonate from the blood known as metabolic acidosis, or due to a buildup of carbon dioxide in the blood due to poor lung function, known as respiratory acidosis. It is the opposite of alkalosis, which is a condition in which there is too much base in the body fluids.
Free Access on TEAS 7 Exams and Study Notes
- Access to all TEAS 7 Exams
- Performance Tracking and Analysis
- Well Documented and Explained Questions and Answers
- 2000+ Questions and Correct Answers: Answers Well Explained
- Libary of Detailed StudyNotes
- Topical Questions and Answers on Examinable topics
TEAS 7 Exams (Q&A)
TEAS 7 Study Notes
TEAS 7 Topical Tests

TEAS 7 Study Guides
Quick Links
Refer a Friend
Refer a friend and claim free unlimited access

© 2024 ExamGates Made with by ExamGates
