Which of the following atoms is a cation?
A. 14 protons, 14 neutrons, 18 electrons
B. 34 protons, 45 neutrons, 36 electrons
C. 35 protons, 44 neutrons, 35 electrons
D. 82 protons, 125 neutrons, 78 electrons
Because it has more protons than electrons, this atom has a positive charge and can be classified as a cation. When a metal such as sodium reacts to become stable, it loses its valence electrons. At first, it is a neutral atom with 11 protons and 11 electrons. When it loses an electron, the number of protons does not change, and the atom has 11 protons and 10 electrons. Because there is one more positively charged proton, a cation forms. A cation is an ion with a net positive charge.
Therefore, the Correct Answer is D.
More Questions on TEAS 7 Science
-
Q #1: During which of the following phase changes will the cohesion between the particles in a substance decrease?
A. Condensation
B. Deposition
C. Freezing
D. Vaporization
Answer Explanation
If the cohesion between particles decreases, then the particles must be undergoing a phase change that allows particles to move farther apart. This happens when a substance vaporizes and turns from liquid to gas. Any phase change that moves to the right in the diagram above requires energy to be added to the system because the substance has more energy at the end of the phase change. The phase changes are melting, vaporization (boiling), and sublimation. When energy is added, particles move faster and can break away from each other more easily as they move to a state of matter with a higher amount of energy. This is most commonly done by heating the substance.
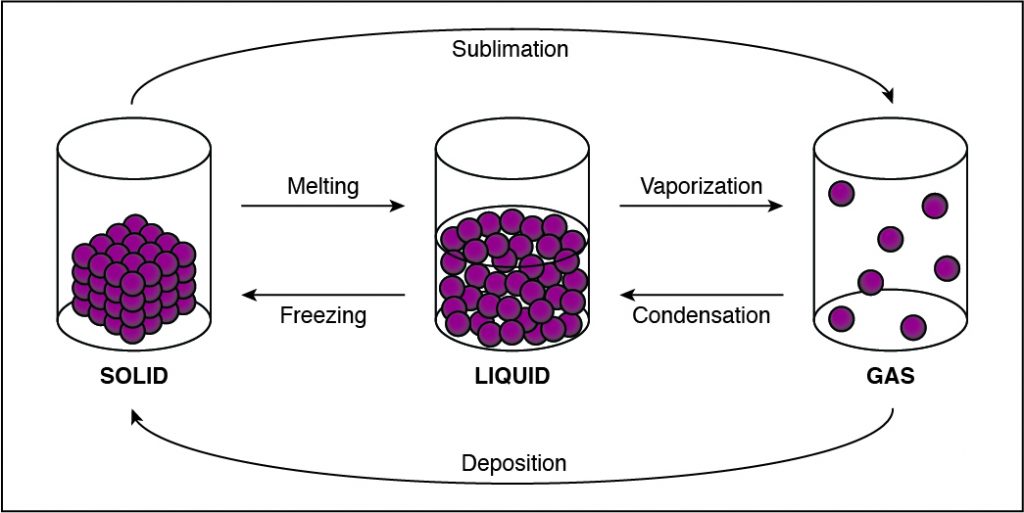
-
Q #2: After food has been masticated in the oral cavity, where does it go next?
A. Colon
B. Liver
C. Pancreas
D. Pharynx
Answer Explanation
Once the food has been masticated in the oral cavity (mouth), it is then swallowed and travels back into the pharynx down into the esophagus, which leads into the stomach.
-
Q #3: A researcher notices a positive correlation between the height of a plant and nutrient concentration over time. Based on this observation, what conclusion does he reach?
A. The height of a plant increases in the absence and presence of the nutrients
B. When the amount of nutrients available to the plant decreases, its height increases.
C. The amount of nutrients available to a plant is independent of how tall the plant gets
D. When the amount of nutrients available to the plant increases, its height also increases.
Answer Explanation
Because this is a positive correlation, if the nutrient concentration increases or decreases, plant height will either increase or decrease accordingly.
While analyzing data, scientists tend to observe cause-and-effect relationships. These relationships can be quantified using correlations. Correlations measure the amount of linear association between two variables. There are three types of correlations:
Positive correlation:
As one variable increases, the other variable also increases. This is also known as a direct correlation.Negative correlation:
As one variable increases, the other decreases. The opposite is true if one variable decreases. A negative correlation is also known as an inverse correlation or an indirect correlation.No correlation:
There is no connection or relationship between two variables. -
Q #4: Which example is part of the scientific method?
A. A student reads about a new way to harness energy from the sun.
B. A researcher studies the effects of car exhaust on how people breathe.
C. A researcher analyzes how many plants respond well to a new fertilizer
D. A student discovers how insulin plays a role in the development of diabetes
Answer Explanation
One step of the scientific method is to analyze information or data collected from the experiment to conclude whether the hypothesis is supported.
Recall that these make up the scientific method, described below:
- Problem: The question created because of an observation. Example: Does the size of a plastic object affect how fast it naturally degrades in a lake?
- Research: Reliable information available about what is observed. Example: Learn how plastics are made and understand the properties of a lake.
- Hypothesis: A predicted solution to the question or problem. Example: If the plastic material is small, then it will degrade faster than a large particle.
- Experiment: A series of tests used to evaluate the hypothesis. Experiments consist of an independent variable that the researcher modifies and a dependent variable that changes due to the independent variable. They also include a control group used as a standard to make comparisons.
- Example: Collect plastic particles both onshore and offshore of the lake over time. Determine the size of the particles and describe the lake conditions during this time period.
- Observe: Analyze data collected during an experiment to observe patterns.
- Example: Analyze the differences between the numbers of particles collected in terms of size.
- Conclusion: State whether the hypothesis is rejected or accepted and summarize all results.
- Communicate: Report findings so others can replicate and verify the results.
-
Q #5: A person is diagnosed as having acidosis, a condition in which the blood pH is below 7.45. What does the doctor most likely conclude?
A. Too much carbon dioxide is found in the blood.
B. Highly oxygenated blood circulates through the body
C. A blockage prevents blood from leaving the pulmonary artery
D. The nasal cavity has a difficult time clearing particles from the air.
Answer Explanation
Acidosis is when the body fluids contain too much acid, or low pH. The kidneys and lungs are unable to keep the body’s pH in balance. Acidosis is the result when there is too much loss of bicarbonate from the blood known as metabolic acidosis, or due to a buildup of carbon dioxide in the blood due to poor lung function, known as respiratory acidosis. It is the opposite of alkalosis, which is a condition in which there is too much base in the body fluids.
-
Q #6: Which is classified as a type of acid-base reaction that produces a salt?
A. Combination
B. Decomposition
C. Hydrolysis
D. Neutralization
Answer Explanation
A neutralization reaction is a type of acid-base reaction where an acid and base react to form a salt and water.
In an aqueous solution, a base increases the hydroxide concentration (OH–), while an acid increases the hydrogen ion (H+) concentration. Sometimes, neutralization reactions also occur. This type of reaction happens when an acid and a base react with each other to form water and salt. Salt is typically defined as an ionic compound that includes any cation except H+ and any anion except OH–. Consider the following example of a neutralization reaction between hydrobromic acid (HBr) and potassium hydroxide (KOH).
HBr+KOH→KBr+H2O
Not all neutralization reactions proceed in the manner where all reactants are in the aqueous phase. In some chemical reactions, one reactant may be a solid. The neutralization reaction can still proceed to completion.
-
Q #7: What is the final structure through which urine must travel to empty out of the body?
A. Bladder
B. Kidney
C. Ureter
D. Urethra
Answer Explanation
The primary organ of the urinary system is the kidney. Blood from the heart flows through the kidneys via the renal artery. As blood drains from the kidney, it exits through a series of veins, the most prominent of which is the renal vein. When urine is produced, it does not drain through the tubes through which blood flows. Rather, urine flows through two ureters before emptying into the urinary bladder.
The following steps outline how the urinary system works:
- Kidney filters and excretes wastes from blood, producing urine.
- Urine flows down the ureters.
- Urine empties into the bladder and is temporarily stored.
- Bladder, when filled, empties urine out of the body via the urethra.
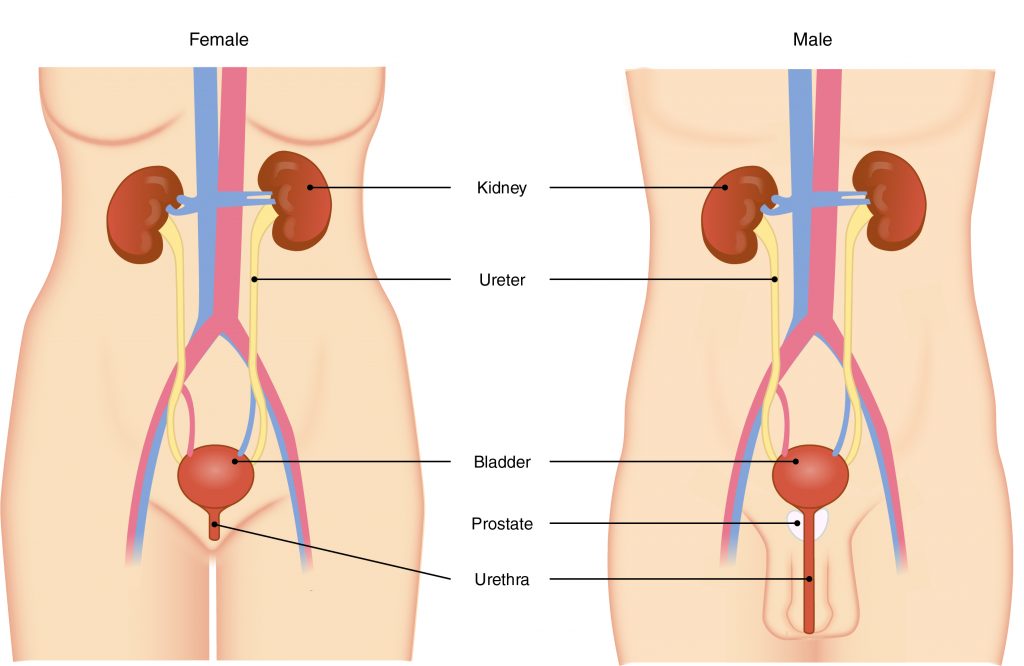
-
Q #8: What phase is the cell cycle part of?
A. Interphase
B. Metaphase
C. Prophase
D. Telophase
Answer Explanation
Before mitosis or meiosis occurs, interphase must happen. This is when the cell cycle takes place. The cell cycle is an organized process divided into two phases: interphase and the M (mitotic) phase. During interphase, the cell grows and copies its DNA. After the cell reaches the M phase, division of the two new cells can occur. The G1, S, and G2 phases make up interphase.
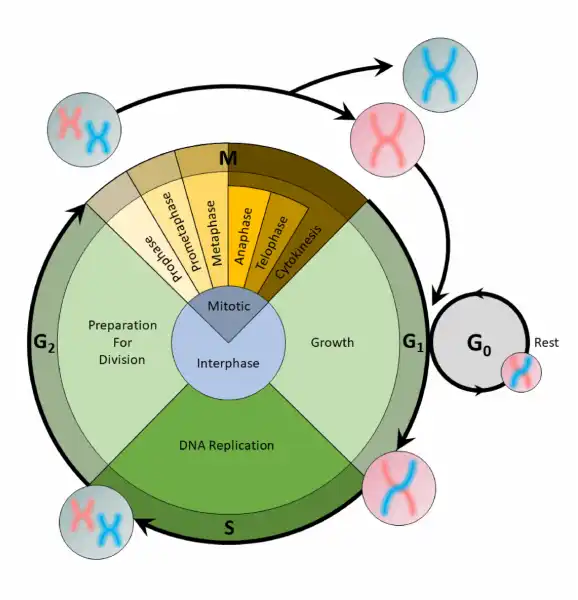
-
Q #9: An intracellular chemical signal can be produced in the cell membrane. Once it is produced, where does it go?
A. To a different cell
B. To another part of the same cell
C. To a region right outside the cell
D. To an area with a high ion concentration
Answer Explanation
There are two major types of receptor molecules that respond to an intercellular chemical signal:
- Intracellular receptors: These receptors are located in either the cytoplasm or the nucleus of the cell. Signals diffuse across the cell membrane and bind to the receptor sites on intracellular receptors, of the same cell.
- Membrane-bound receptors: These receptors extend across the cell membrane, with their receptor sites on the outer surface of the cell membrane. They respond to intercellular chemical signals that are large, water-soluble molecules that do not diffuse across the cell membrane.
-
Q #10: What solution has a pH of 7?
A. Aniline
B. Pyridine
C. Pure water
D. Sodium hydroxide
Answer Explanation
A pH of 7 is a neutral solution, which is how pure water is classified. Researchers can determine the strength of an acid or a base by measuring the pH of a solution. The pH value describes how acidic or basic a solution is. On pH scale, shown below, if the number is less than 7 the solution is acidic. A pH greater than 7 means the solution is basic. When the pH is exactly 7, the solution is neutral.
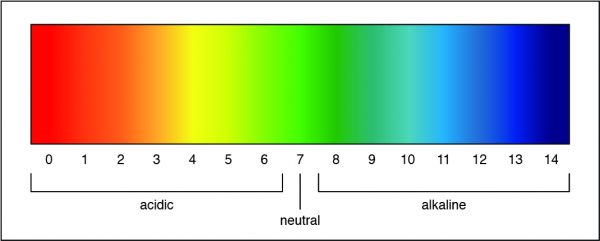
Free Access on TEAS 7 Exams and Study Notes
- Access to all TEAS 7 Exams
- Performance Tracking and Analysis
- Well Documented and Explained Questions and Answers
- 2000+ Questions and Correct Answers: Answers Well Explained
- Libary of Detailed StudyNotes
- Topical Questions and Answers on Examinable topics
TEAS 7 Exams (Q&A)
TEAS 7 Study Notes
TEAS 7 Topical Tests

TEAS 7 Study Guides
Quick Links
Refer a Friend
Refer a friend and claim free unlimited access

© 2024 ExamGates Made with by ExamGates
