Which of the following can lead to a tsunami?
A. Sunspot activity
B. Lightning strikes.
C. Earthquakes
D. Flooding.
A tsunami is a catastrophic ocean wave that is usually caused by a submarine earthquake.
It can also be caused by an underwater or coastal landslide, the eruption of a volcano, or the impact of a meteor or comet in a body of water.
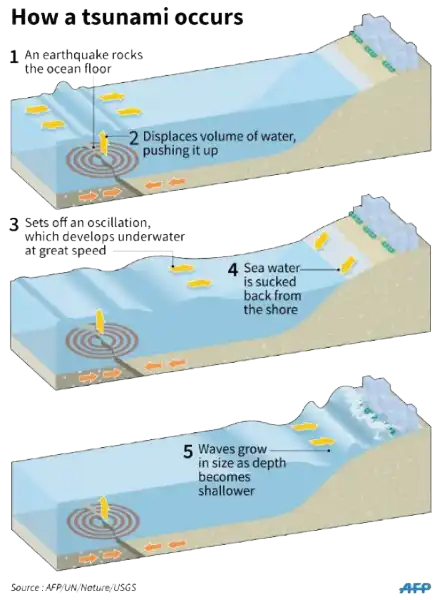
Choice A is not correct because sunspot activity does not cause tsunamis.
Choice B is not correct because lightning strikes do not cause tsunamis.
Choice D is not correct because flooding does not cause tsunamis.
Therefore, the Correct Answer is C.
More Questions on TEAS 7 Science
-
Q #1: Which of the following summarizes a change that takes place as a solid turns to a liquid?
A. Particles become less ordered.
B. Particles have a decrease in mobility.
C. Particles move closer together
D. Intermolecular forces between particles become stronger.
Answer Explanation
As a solid turns to a liquid, the particles become less ordered and more free to move around.
Choice B is not correct because particles have an increase in mobility as a solid turns to a liquid.
Choice C is not correct because particles move further apart as a solid turns to a liquid.
Choice D is not correct because intermolecular forces between particles become weaker as a solid turns to a liquid.
-
Q #2: Which of the following physiological responses is caused by the release of antidiuretic hormone?
A. Increase in the concentration of calcium in the glomerulus.
B. Increase in water reabsorption in the collecting duct
C. Decrease in the concentration of calcium in the glomerulus.
D. Decrease in water reabsorption in the collecting duct
Answer Explanation
Antidiuretic hormone (ADH), also known as vasopressin, is a hormone that helps regulate the amount of water in your body.
It works to control the amount of water your kidneys reabsorb as they filter out waste from your blood.
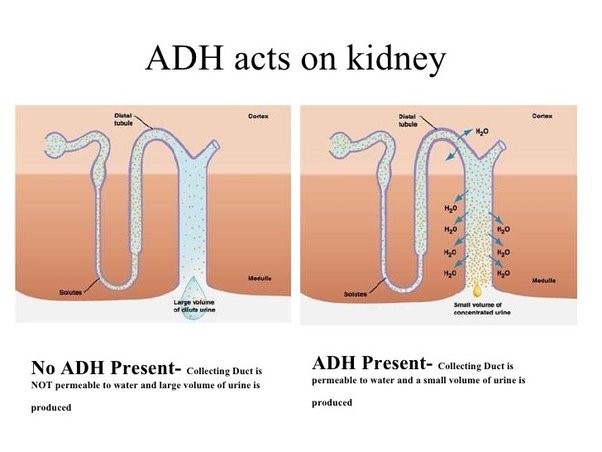
Choice A is not correct because an increase in the concentration of calcium in the glomerulus is not a physiological response caused by the release of antidiuretic hormone.
Choice C is not correct because a decrease in the concentration of calcium in the glomerulus is not a physiological response caused by the release of antidiuretic hormone.
Choice D is not correct because a decrease in water reabsorption in the collecting duct is not a physiological response caused by the release of antidiuretic hormone.
-
Q #3: In a phase diagram, which of the following is the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously?
A. Triple point
B. Critical temperature
C. Critical point
D. Absolute zero
Answer Explanation
Triple point.
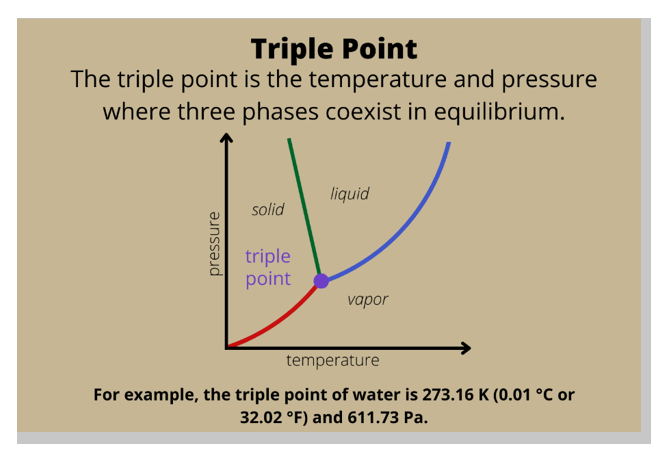
In a phase diagram, the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously is the triple point.
The triple point is a unique point on a phase diagram where the three states of matter (solid, liquid, and gas) can coexist in equilibrium.
At the triple point, the temperature and pressure of the substance are fixed.
Option B, critical temperature, is the temperature at which a gas cannot be liquefied, regardless of the pressure applied.
It is a characteristic property of a substance and is typically higher than the boiling point of the liquid at standard pressure.
Option C, critical point, is the point on a phase diagram where the liquid and gas phases of a substance become indistinguishable.
At the critical point, the distinction between the liquid and gas phases disappears, and the substance becomes a supercritical fluid.
Option D, absolute zero, is the theoretical temperature at which all matter has zero thermal energy.
At absolute zero, all substances are in their solid state, but it is not relevant to a phase diagram, as it is a temperature where no transitions between states occur.
In summary, the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously in a phase diagram is the triple point, whereas the other options provided are not relevant or are characteristic properties of substances in different contexts.
-
Q #4: Which of the following vessels carries oxygenated blood?
A. Superior vena cava
B. Inferior vena cava.
C. Pulmonary artery
D. Pulmonary vein
Answer Explanation
The pulmonary veins are the vessels that carry oxygenated blood from the lungs to the left atrium of the heart.
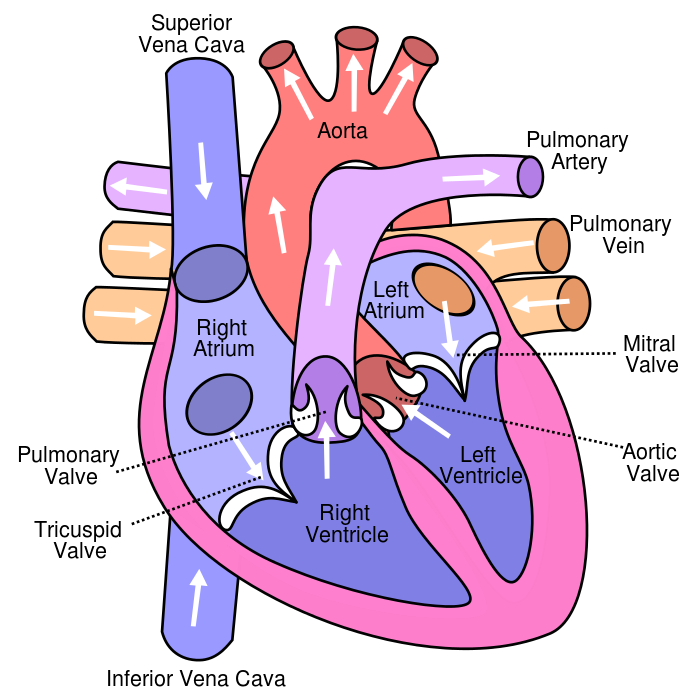
Choice A is not correct because the superior vena cava carries deoxygenated blood from the upper body to the right atrium of the heart.
Choice B is not correct because the inferior vena cava carries deoxygenated blood from the lower body to the right atrium of the heart.
Choice C is not correct because the pulmonary artery carries deoxygenated blood from the right ventricle of the heart to the lungs.
-
Q #5: Which of the following ions binds to the troponin complex, initiating contraction of a muscle?
A. Potassium.
B. Calcium.
C. Phosphorus.
D. Sodium
Answer Explanation
Calcium ions play a crucial role in initiating muscle contraction.
When a muscle cell is stimulated to contract by an action potential, calcium channels open in the sarcoplasmic membrane and release calcium into the sarcoplasm.
Some of this calcium attaches to troponin, which causes it to change shape.
This shape change exposes binding sites for myosin on the actin filaments.
Myosin’s binding to actin causes crossbridge formation, and contraction of the muscle begins.
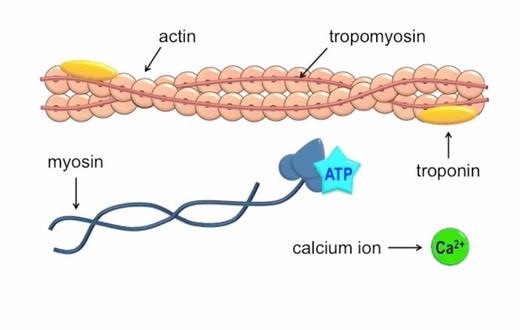
The other ions mentioned in the question do not have this specific role in muscle contraction.
Potassium ions are important for maintaining the resting membrane potential of cells, but they do not bind to the troponin complex.
Phosphorus ions are important for energy metabolism, but they do not bind to the troponin complex.
Sodium ions are important for generating action potentials, but they do not bind to the troponin complex.
-
Q #6: Which of the following organic molecules contain both an amine and carboxyl group?
A. Lipids
B. Chitin
C. Cellulose
D. Proteins
Answer Explanation
Proteins.
Proteins are made up of amino acids which are organic molecules that contain both an amine functional group (–NH2) and a carboxylic acid functional group (– COOH).
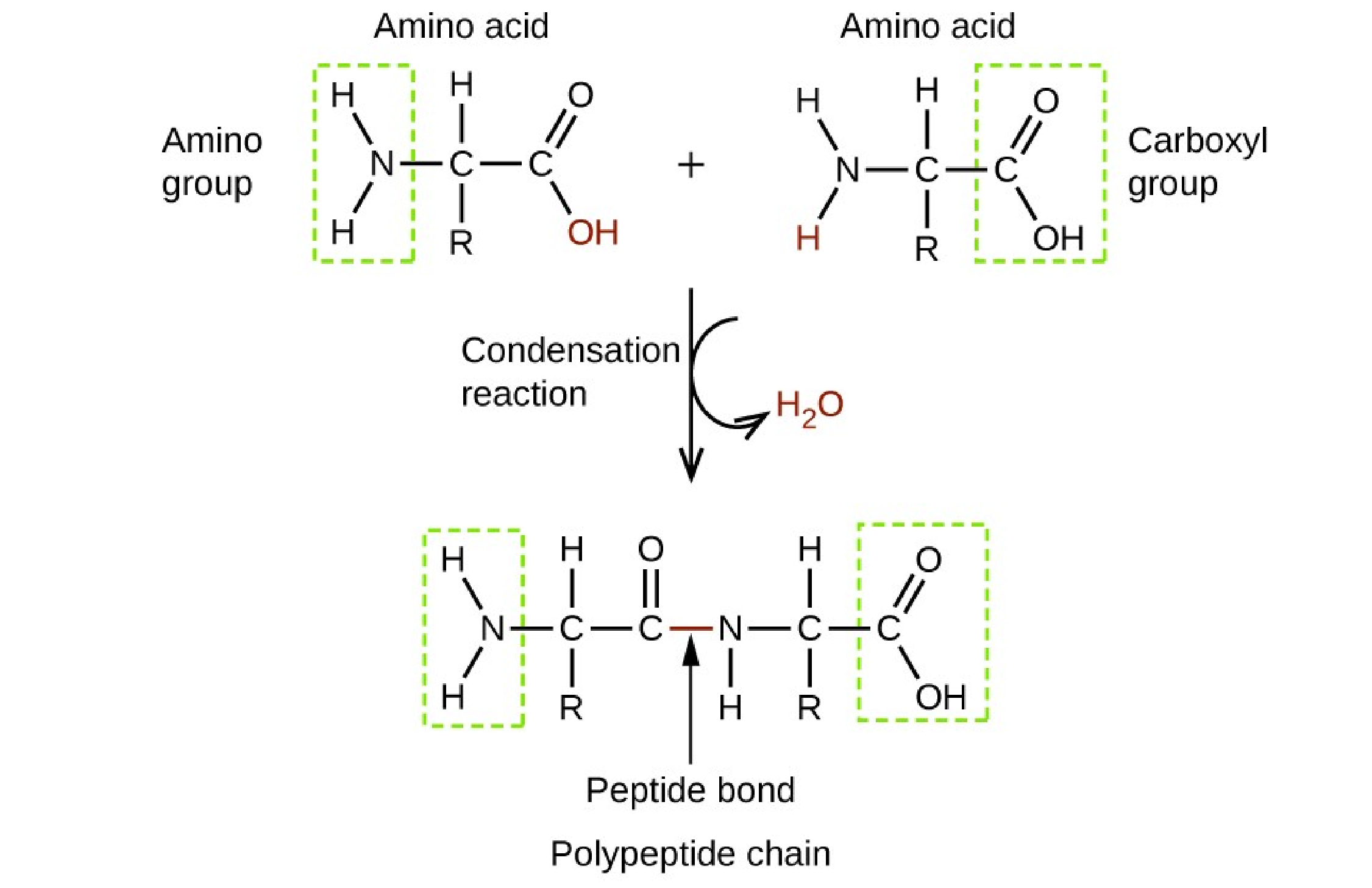
Choice A, Lipids, is not the correct answer because lipids are a group of naturally occurring molecules that include fats, waxes, sterols, fat-soluble vitamins, monoglycerides, diglycerides, triglycerides, phospholipids, and others.
They do not contain both an amine and carboxyl group.
Choice B, Chitin, is not the correct answer because chitin is a long-chain polymer of N-acetylglucosamine, a derivative of glucose.
It does not contain both an amine and carboxyl group.
Choice C, Cellulose, is not the correct answer because cellulose is an organic compound with the formula (C6H10O5)n, a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units.
It does not contain both an amine and carboxyl group.
-
Q #7: Which of the following is correct regarding the pH scale?
A. A substance with a pH of 3 is two times more alkaline than a substance with a pH of 4
B. A substance with a pH of 3 is two times more acidic than a substance with a pH of 4
C. A substance with a pH of 3 is 10 times more acidic than a substance with a pH of 4
D. A substance with a pH of 3 is 10 times more alkaline than a substance with a pH of 4
Answer Explanation
The pH scale is a logarithmic scale that measures the acidity or alkalinity of a solution.
A solution with a pH of 7 is neutral, while a solution with a pH less than 7 is acidic and a solution with a pH greater than 7 is alkaline.
Because the pH scale is logarithmic, each whole number change in pH represents a tenfold change in acidity or alkalinity.
Therefore, a substance with a pH of 3 is 10 times more acidic than a substance with a pH of 4.
Choice A.
A substance with a pH of 3 is two times more alkaline than a substance with a pH of 4 is not correct because it incorrectly states that the substance with a lower pH is more alkaline and also incorrectly states the magnitude of the difference in acidity or alkalinity.
Choice B.
A substance with a pH of 3 is two times more acidic than a substance with a pH of 4 is not correct because it correctly states that the substance with a lower pH is more acidic but incorrectly states the magnitude of the difference in acidity.
Choice D.
A substance with a pH of 3 is 10 times more alkaline than a substance with a pH of 4 is not correct because it incorrectly states that the substance with a lower pH is more alkaline.
-
Q #8: Which of the following properties of water explains its solvent abilities for certain substances?
A. Kinetic energy of liquid water molecules
B. High specific heat
C. High surface tension
D. Polarity of water molecules
Answer Explanation
The polarity of water molecules explains its solvent abilities for certain substances.
Water is a polar molecule because it has a partial positive charge on one end and a partial negative charge on the other end due to the unequal sharing of electrons between the oxygen and hydrogen atoms.
This polarity allows water to dissolve other polar substances and ionic compounds.
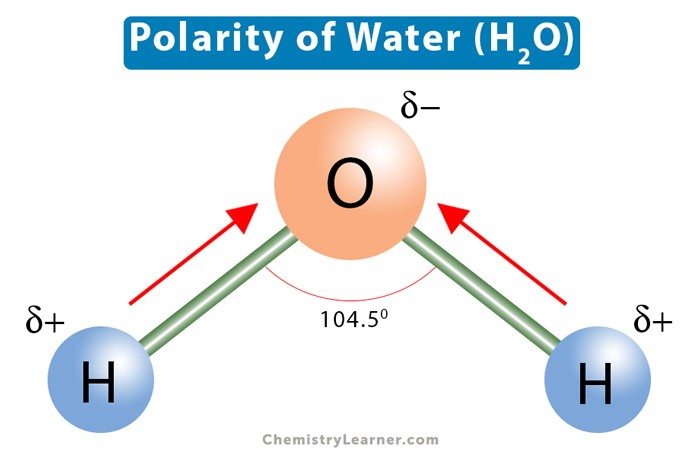
Choice A.
Kinetic energy of liquid water molecules is not the correct answer because kinetic energy refers to the energy of motion and does not directly explain water’s solvent abilities.
Choice B.
High specific heat is not the correct answer because specific heat refers to the amount of heat required to raise the temperature of a substance and does not directly explain water’s solvent abilities.
Choice C.
High surface tension is not the correct answer because surface tension refers to the cohesive forces between liquid molecules and does not directly explain water’s solvent abilities.
-
Q #9: Which of the following represents the complementary strand of a DNA sequence 3' TCGATCGCA 5'?
A. 3' AGCTAGCGT 5'
B. 5’ AGCTAGCGT 3’
C. 5' UCGAUCGCA 3'
D. 3' TCGUTCGCU 3'
Answer Explanation
In DNA, the nitrogenous bases adenine (A) and thymine (T) pair together, while cytosine (C) and guanine (G) pair together.
Therefore, the complementary strand of the given DNA sequence 3' TCGATCGCA 5' would have the complementary nitrogenous bases as:
5’ AGCTAGCGT 3’
NOTE: The 5’ to 3’ direction of the complementary strand is opposite to that of the given strand.
Choice A.
3’ AGCTAGCGT 5’ is not correct because it is not complementary to the given strand.
Choice C.
5’ UCGAUCGCA 3’ is not correct because it contains uracil (U), which is a nitrogenous base found in RNA, not DNA.
Choice D.
3’ TCGUTCGCU 3’ is not correct because it also contains uracil (U), which is a nitrogenous base found in RNA, not DNA.
-
Q #10: Which of the following results in osteoporosis?
A. An increase in osteocyte activity while osteoclast activity continues at expected levels.
B. A decline in osteoclast activity while osteoblast activity continues at expected levels.
C. An increase in osteocyte activity while osteoblast activity reduces.
D. A decline in osteoblast activity while osteoclast activity continues at expected levels.
Answer Explanation
A decline in osteoblast activity while osteoclast activity continues at expected levels results in osteoporosis.
Osteoporosis is caused by an imbalance between the functioning of osteoclast and osteoblast cells.
Osteoblasts are responsible for forming new bone, while osteoclasts break down old bone.
If osteoblast activity declines while osteoclast activity continues at expected levels, this means that more bone is being broken down than is being formed, leading to a loss of bone density and an increased risk of osteoporosis.
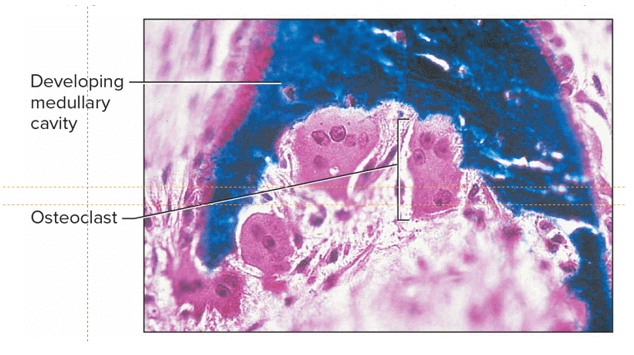
Choice A is incorrect because an increase in osteocyte activity would not result in osteoporosis.
Osteocytes are mature bone cells that maintain the mineral concentration of the bone matrix.
Choice B is incorrect because a decline in osteoclast activity would not result in osteoporosis.
Osteoclasts break down old bone, so a decline in their activity would mean that less bone is being broken down.
Choice C is incorrect because an increase in osteocyte activity would not result in osteoporosis.
As mentioned earlier, osteocytes are mature bone cells that maintain the mineral concentration of the bone matrix.
Free Access on TEAS 7 Exams and Study Notes
- Access to all TEAS 7 Exams
- Performance Tracking and Analysis
- Well Documented and Explained Questions and Answers
- 2000+ Questions and Correct Answers: Answers Well Explained
- Libary of Detailed StudyNotes
- Topical Questions and Answers on Examinable topics
TEAS 7 Exams (Q&A)
TEAS 7 Study Notes
TEAS 7 Topical Tests

TEAS 7 Study Guides
Quick Links
Refer a Friend
Refer a friend and claim free unlimited access

© 2024 ExamGates Made with by ExamGates
