Which of the following correctly orders structures from simple to complex?
A. Cells, tissues, atoms, organs
B. Atoms, organs, tissues, cells
C. Atoms, cells, tissues, organs
D. Organs, tissues, cells, atoms
The correct answer is c. Atoms, cells, tissues, organs. This is the correct order of structures from simple to complex. Atoms are the smallest and simplest units of mater. Cells are made up of atoms and are the basic units of life.
Tissues are groups of similar cells that work together to perform a specific function. Organs are made up of different types of tissues and perform more complex functions.
A. Cells, tissues, atoms, organs is not the correct order from simple to complex.
B. Atoms, organs, tissues, cells is not the correct order from simple to complex.
D. Organs, tissues, cells, atoms is not the correct order from simple to complex.
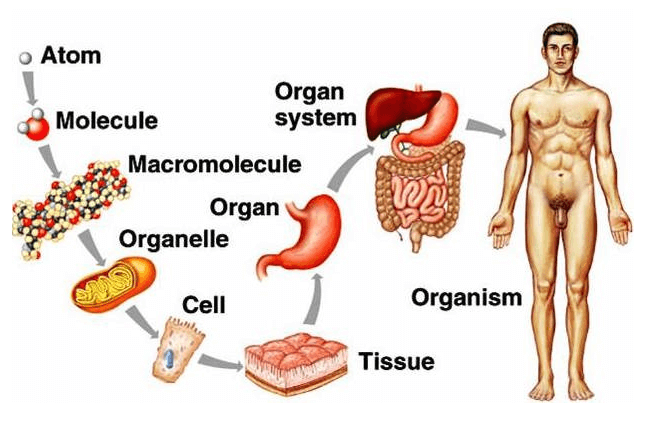
Therefore, the Correct Answer is C.
More Questions on TEAS 7 Science
-
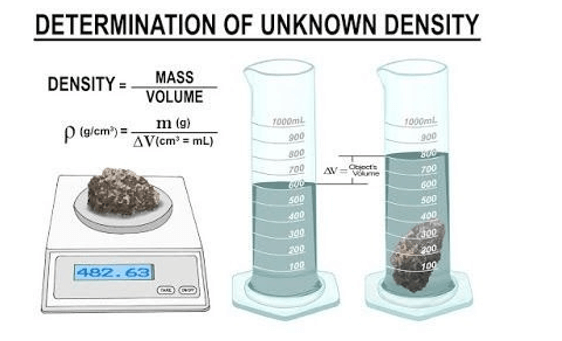
Q #1: To accurately measure the density of a series of small irregular solids made of plastic, wood, fibreglass, and glass, a student will need which of the following laboratory tools?
A. Graduated cylinder, water, weighing balance
B. Graduated cylinder, spectrophotometer, water
C. Graduated beaker, metric ruler, water
D. Weighing balance, Bunsen burner, metric ruler
Answer Explanation
To accurately measure the density of a series of small irregular solids made of plastic, wood, fiberglass, and glass, a student will need a graduated cylinder, water, and a weighing balance. The student can use the water displacement method to determine the volume of each solid by measuring the volume of water displaced when the solid is submerged in a graduated cylinder filled with water. The mass of each solid can be measured using a weighing balance. The density can then be calculated by dividing the mass by the volume.
The other options are not correct because they do not provide the necessary tools to accurately measure the density of the solids. A spectrophotometer is used to measure light absorption and is not necessary for measuring density. A graduated beaker is less accurate than a graduated cylinder for measuring volume. A Bunsen burner is used for heating and is not necessary for measuring density.
-
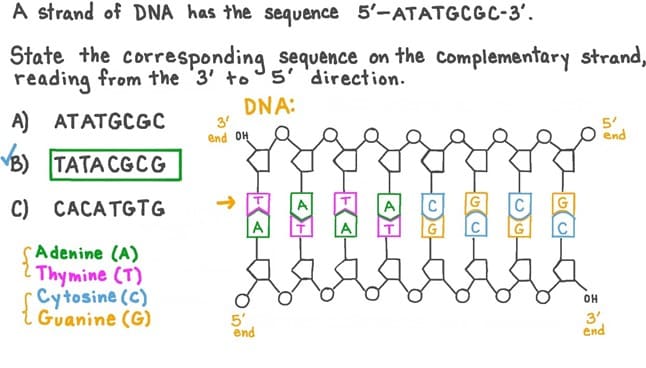
Q #2: If a portion of a strand of DNA bases reads 3’TCGATCGCA 5’, what would the sequence of bases on the complementary strand read?
A. 5’AGCTAGCGT 3’
B. 3’ TCGUTCGCU 5’
C. 3’ AGCTAGCGT 5’
D. 5’ GGUTACTAC 3’
Answer Explanation
The sequence of bases on the complementary strand of DNA would read 5’AGCTAGCGT 3’ (Choice A). In DNA, the nitrogenous bases adenine (A) and thymine (T) pair together, and cytosine (C) and guanine (G) pair together. The complementary strand is also antiparallel to the original strand, meaning that it runs in the opposite direction with the 5' end matching up with the 3' end of the original strand.
The other options do not accurately represent the complementary sequence of bases or the antiparallel orientation of the strands.
BONUS:
-
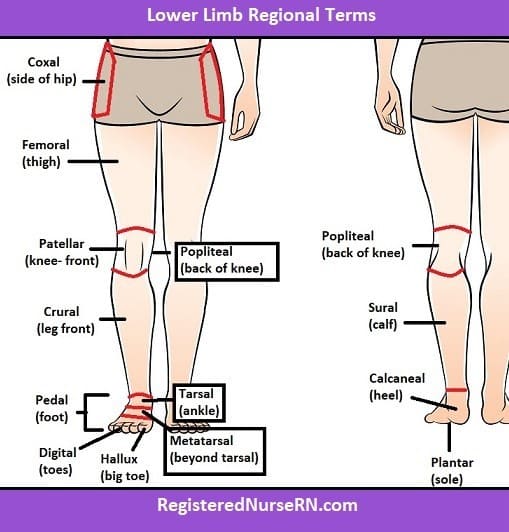
Q #3: In which of the following regions of the body are the tibia and fibula?
A. Coxal
B. Antecubital
C. Tarsal
D. Crural
Answer Explanation
The tibia and fibula are located in the crural region of the body, which is the lower leg between the knee and ankle. The coxal region refers to the hip area, the antecubital region is the front of the elbow, and the tarsal region is the ankle and foot.
-
Q #4: Which of the following substances is responsible for donating H+ ions to act as a buffer when blood pH rises?
A. Oxygen
B. Carbon monoxide
C. Carbon dioxide
D. Carbonic acid
Answer Explanation
The correct answer is d. Carbonic acid. When blood pH rises, carbonic acid can donate H+ ions to act as a buffer and help maintain the pH within a normal range. Carbonic acid is formed when carbon dioxide dissolves in water and reacts with it.
A. Oxygen is not responsible for donating H+ ions to act as a buffer when blood pH rises.
B. Carbon monoxide is a toxic gas that does not play a role in buffering blood pH.
C. Carbon dioxide can dissolve in water to form carbonic acid, which can then donate H+ ions to act as a buffer.
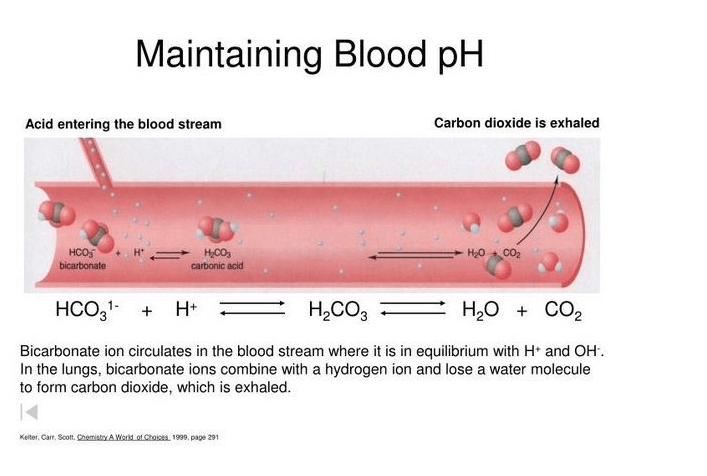
-
Q #5: Which of the following growth curves shows a population that is at its carrying capacity?
A. B
B. C
C. A
D. D
Answer Explanation
A population is said to be at its carrying capacity when it has reached the maximum number of individuals that can be sustained in a particular environment over a prolonged period of time, given the available resources and the prevailing environmental conditions.
In other words, carrying capacity refers to the maximum population size that a given ecosystem can support without being depleted of resources or experiencing environmental degradation. Once a population reaches its carrying capacity, its growth rate slows down and stabilizes, as individuals start to compete more intensely for resources such as food, water, and shelter, and mortality rates increase.
Carrying capacity is an important concept in ecology and population biology because it helps to explain the dynamics of natural populations and how they are influenced by changes in the environment, such as climate change, habitat loss, and human activities.
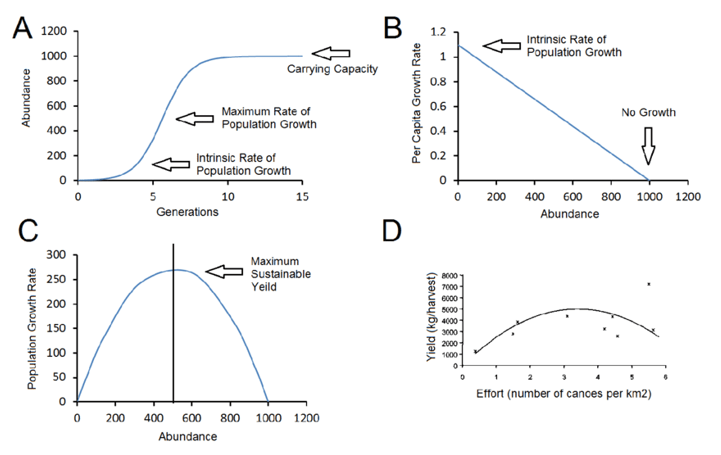
-
Q #6: The mitochondrial inner membrane carries out the same function in cellular respiration as the ________ membrane of chloroplasts in photosynthesis. Which of the following correctly completes the sentence above?
A. Thylakoid
B. Epithelial
C. Nuclear
D. Tonoplast
Answer Explanation
The thylakoid membrane of chloroplasts is where the light-dependent reactions of photosynthesis take place, while the mitochondrial inner membrane is where the electron transport chain and ATP synthesis occur during cellular respiration.
The tonoplast is the membrane that surrounds the central vacuole in plant cells. It is not involved in cellular respiration or photosynthesis.
The other options, epithelial and nuclear, are not related to these processes.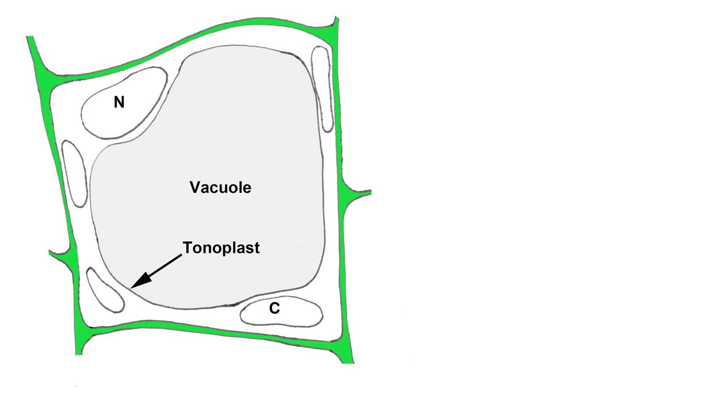
-
Q #7: A study found two processes, Process A and Process B, to be correlated. Which of the following is true for these processes?
A. The study indicates that Process A causes Process B.
B. The study cannot indicate whether Process A and B have a positive relationship.
C. The study does not indicate a causal relationship between the processes.
D. The study cannot indicate whether Process A and B have a negative relationship.
Answer Explanation
The correct answer is c.
The study does not indicate a causal relationship between the processes. A correlation between two processes means that there is a statistical relationship between them, but it does not necessarily imply causation. In other words, just because two processes are correlated does not mean that one causes the other.
b.The study does not indicate that Process A causes Process B.
b.The study can indicate whether Process A and B have a positive relationship if the correlation is positive.
d. The study can indicate whether Process A and B have a negative relationship if the correlation is negative.
-
Q #8: How many times stronger is the hydrogen-ion concentration of a pH 4 solution as compared with a pH 9 solution?
A. 0.00001
B. 5
C. 100,000
D. 50
Answer Explanation
The correct answer is c. 100,000. The pH scale is a logarithmic scale, which means that each change of one pH unit represents a tenfold change in the hydrogen-ion concentration. A pH 4 solution has a hydrogen-ion concentration that is 10^5 (or 100,000) times greater than that of a pH 9 solution.
a. 0.00001 is the hydrogen-ion concentration of a pH 9 solution as compared with a pH 4 solution.
b. 5 is the difference in pH units between a pH 4 solution and a pH 9 solution.
d. 50 is not the correct answer.
-
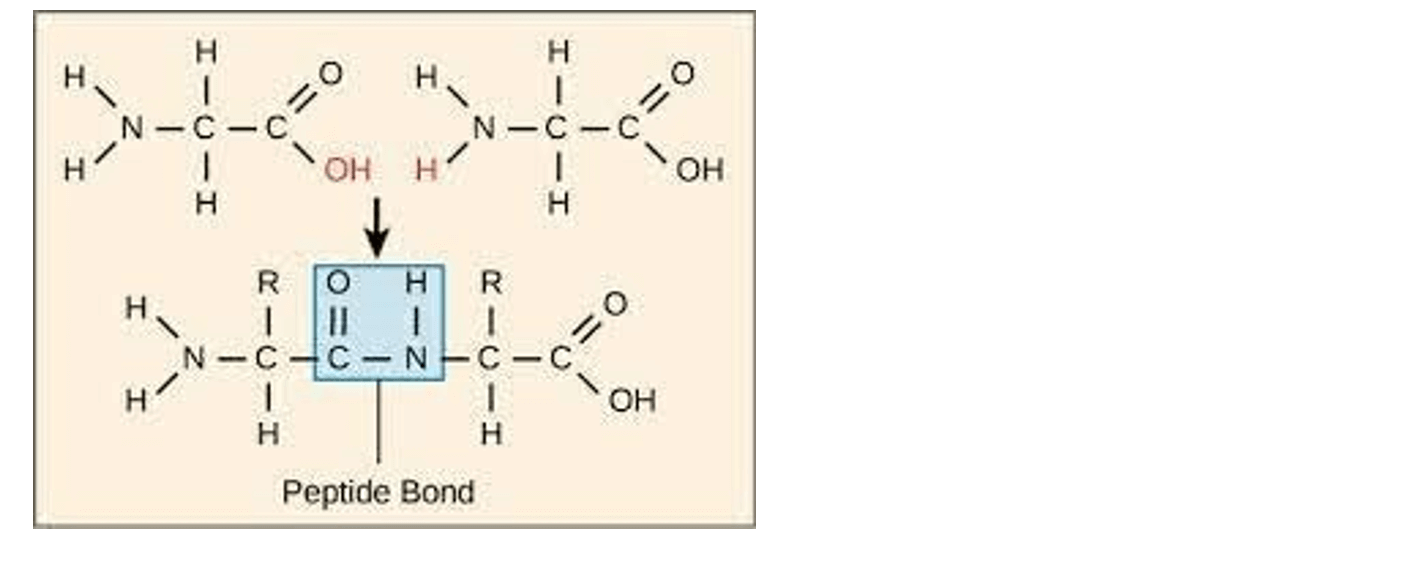
Q #9: The covalent bonds between the monomers of an enzyme macromolecule are:
A. Ester bonds
B. Peptide bonds
C. Phosphodiester bonds
D. Glycosidic bonds
Answer Explanation
The correct answer is b. Peptide bonds. Enzymes are proteins, and proteins are made up of amino acid monomers linked together by peptide bonds. A peptide bond is a covalent bond that forms between the carboxyl group of one amino acid and the amino group of another amino acid.
a. Ester bonds are covalent bonds that form between a carboxylic acid and an alcohol.
c. Phosphodiester bonds are covalent bonds that form between a phosphate group and two hydroxyl
groups.
d. Glycosidic bonds are covalent bonds that form between two monosaccharides.
-
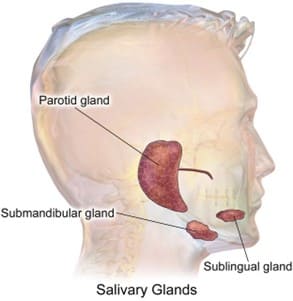
Q #10: Which of the following structures is an exocrine gland?
A. Pineal gland
B. Parathyroid gland
C. Parotid gland
D. Pituitary gland
Answer Explanation
The parotid gland is an exocrine gland that secretes saliva into the mouth. Exocrine glands secrete their products into ducts that carry the secretions to the body's surface or into body cavities. The other options are endocrine glands, which secrete hormones directly into the bloodstream. The pineal gland secretes melatonin, the parathyroid glands secrete parathyroid hormone, and the pituitary gland secretes several hormones that regulate various bodily functions.
Free Access on TEAS 7 Exams and Study Notes
- Access to all TEAS 7 Exams
- Performance Tracking and Analysis
- Well Documented and Explained Questions and Answers
- 2000+ Questions and Correct Answers: Answers Well Explained
- Libary of Detailed StudyNotes
- Topical Questions and Answers on Examinable topics
TEAS 7 Exams (Q&A)
TEAS 7 Study Notes
TEAS 7 Topical Tests

TEAS 7 Study Guides
Quick Links
Refer a Friend
Refer a friend and claim free unlimited access

© 2024 ExamGates Made with by ExamGates
