Which of the following is supported by the cell theory?
A. Cells are alive and recognized as the building blocks for life.
B. Scientists can identify and differentiate cells by using a microscope
C. Cells are produced from existing cells using meiosis instead of mitosis.
D. Living things are composed of a single cell that remains undifferentiated
After scientists were able to view cells under the microscope they formulated the cell theory. One part of this theory concluded that all cells are alive. They also represent the basic unit of life.
All living things are made of cells. Cells are the smallest structural units and basic building blocks of living things. Cells contain everything necessary to keep living things alive. Varying in size and shape, cells carry out specialized functions. This theory, or in-depth explanation, about cells consists of three parts:
- All living things are composed of one or more cells.
- Cells are alive and represent the basic unit of life.
- All cells are produced from pre-existing cells.
Therefore, the Correct Answer is A.
More Questions on TEAS 7 Science
-
Q #1: A researcher notices a positive correlation between the height of a plant and nutrient concentration over time. Based on this observation, what conclusion does he reach?
A. The height of a plant increases in the absence and presence of the nutrients
B. When the amount of nutrients available to the plant decreases, its height increases.
C. The amount of nutrients available to a plant is independent of how tall the plant gets
D. When the amount of nutrients available to the plant increases, its height also increases.
Answer Explanation
Because this is a positive correlation, if the nutrient concentration increases or decreases, plant height will either increase or decrease accordingly.
While analyzing data, scientists tend to observe cause-and-effect relationships. These relationships can be quantified using correlations. Correlations measure the amount of linear association between two variables. There are three types of correlations:
Positive correlation:
As one variable increases, the other variable also increases. This is also known as a direct correlation.Negative correlation:
As one variable increases, the other decreases. The opposite is true if one variable decreases. A negative correlation is also known as an inverse correlation or an indirect correlation.No correlation:
There is no connection or relationship between two variables. -
Q #2: The sequence of amino acids in a gene determines
A. the primary structure of a codon
B. the primary structure of a protein
C. the primary structure of a nucleotide
D. the primary structure of a nucleic acid.
Answer Explanation
The sequence of amino acids in a gene determines the primary structure of a protein. The components necessary for translation are located in the cytoplasm. Translation is the making of proteins by mRNA binding to a ribosome with the start codon that initiates the production of amino acids. A peptide bond forms and connects the amino acids together. The sequence of amino acids determines the protein’s structure, which determines its function.
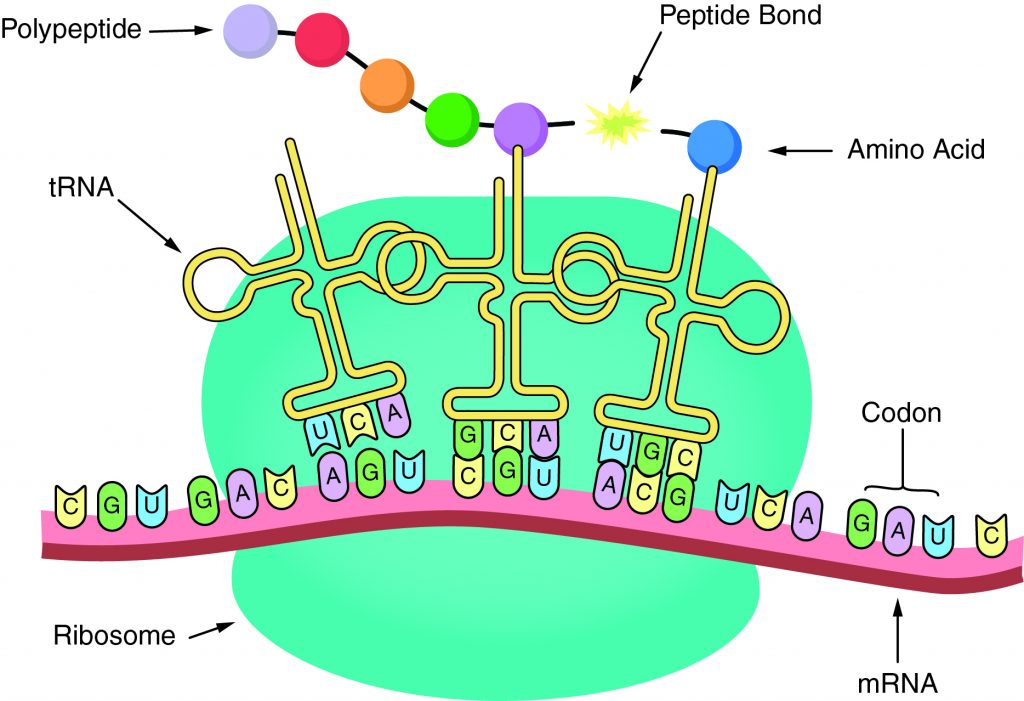
-
Q #3: What solution has a pH of 7?
A. Aniline
B. Pyridine
C. Pure water
D. Sodium hydroxide
Answer Explanation
A pH of 7 is a neutral solution, which is how pure water is classified. Researchers can determine the strength of an acid or a base by measuring the pH of a solution. The pH value describes how acidic or basic a solution is. On pH scale, shown below, if the number is less than 7 the solution is acidic. A pH greater than 7 means the solution is basic. When the pH is exactly 7, the solution is neutral.
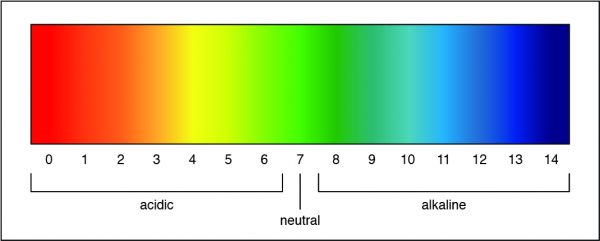
-
Q #4: What is the final structure through which urine must travel to empty out of the body?
A. Bladder
B. Kidney
C. Ureter
D. Urethra
Answer Explanation
The primary organ of the urinary system is the kidney. Blood from the heart flows through the kidneys via the renal artery. As blood drains from the kidney, it exits through a series of veins, the most prominent of which is the renal vein. When urine is produced, it does not drain through the tubes through which blood flows. Rather, urine flows through two ureters before emptying into the urinary bladder.
The following steps outline how the urinary system works:
- Kidney filters and excretes wastes from blood, producing urine.
- Urine flows down the ureters.
- Urine empties into the bladder and is temporarily stored.
- Bladder, when filled, empties urine out of the body via the urethra.
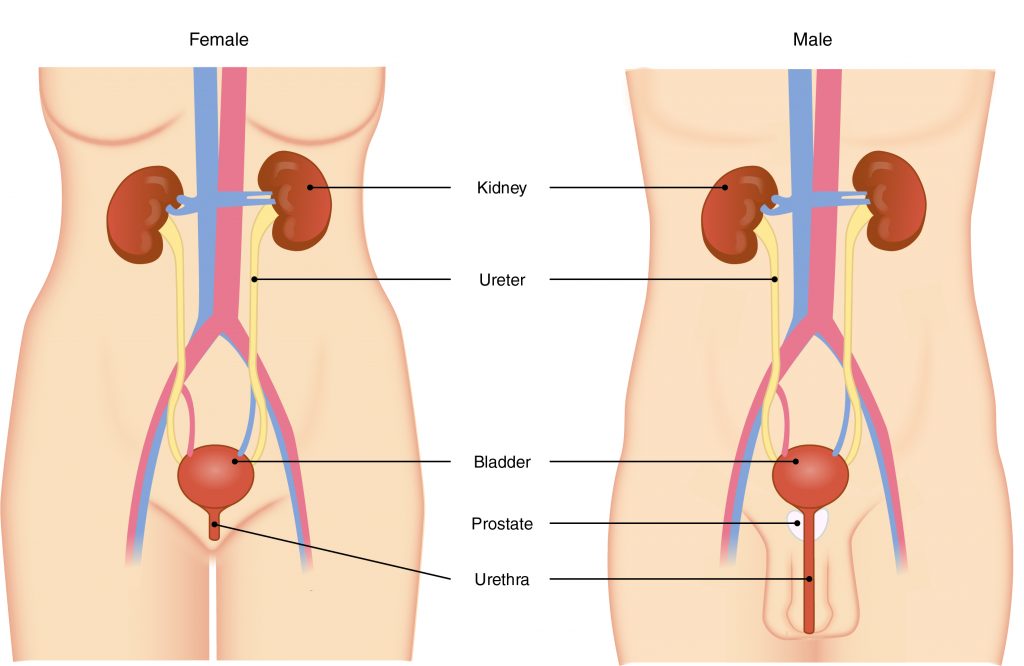
-
Q #5: Which part of the digestive system comes before the stomach?
A. mouth
B. esophagus
C. ileum
D. colon
Answer Explanation
Oral Cavity is the first part of the digestive system. It is bounded by the lips and cheeks and contains the teeth and tongue. Its primary function is to masticate, or chew, and moisten the food.
Pharynx, or throat, connects the mouth to the esophagus.
Esophagus is a muscular tube about 25 centimeters long. Food travels down it to the cardiac sphincter of the stomach.
Pyloric sphincter. The exit of the stomach.
Small intestine is about 6 meters long and consists of three parts: duodenum, jejunum, and ileum.
Large intestine, consists of the cecum, colon, rectum, and anal canal. The cecum is located where the small and large intestine meet. The primary function of the large intestine is to compress the waste and collect any excess water that can be recycled.
Colon is about 1.5 to 1.8 meters long and consists of four parts: the ascending, transverse, descending, and sigmoid colon.
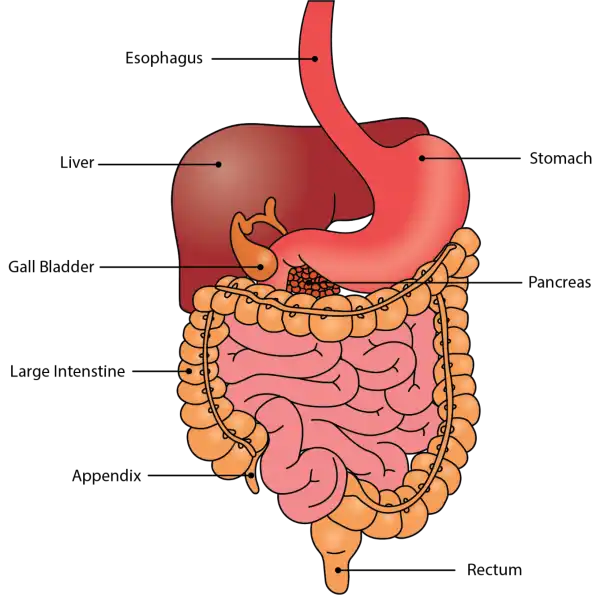
-
Q #6: In the following single-replacement reaction, ______ replaces ______. Cl2+2NaI→2NaCl+I2
A. sodium, iodine
B. chlorine, iodine
C. chlorine, sodium
D. sodium, chlorine
Answer Explanation
In this reaction, chlorine (Cl2) is an element in the reaction that replaces iodine in the compound sodium iodide (NaI). This allows chlorine to form a compound with sodium (NaCl) and leaves iodine (I2) as an element.
Synthesis reactions involve two or more reactants (A and B) combining to form one product (AB). In the example provided, hydrogen (H2) and oxygen (O2) begin as separate elements. At the end of the reaction, the hydrogen and oxygen atoms are bonded in a molecule of water (H2O).
Decomposition reactions have only one reactant (AB) that breaks apart into two or more products (A and B). In the example above, hydrogen peroxide (H2O2) breaks apart into two smaller molecules: water (H2O) and oxygen (O2).
Single-replacement reactions involve two reactants, one compound (AB) and one element (C). In this type of reaction, one element replaces another to form a new compound (AC), leaving one element by itself (B). In the example, zinc replaces hydrogen in hydrochloric acid (HCl). As a result, zinc forms a compound with chlorine, zinc chloride (ZnCl2), and hydrogen (H2) is left by itself.
Double-replacement reactions involve two reactants, both of which are compounds made of two components (AB and CD). In the example, silver nitrate, composed of silver (Ag1+) and nitrate (NO31-) ions, reacts with sodium chloride, composed of sodium (Na1+) and chloride (Cl1-) ions. The nitrate and chloride ions switch places to produce two compounds that are different from those in the reactants.
Combustion reactions occur when fuels burn, and they involve specific reactants and products, as seen in the examples below. Some form of fuel that contains carbon and hydrogen is required. Examples of such fuels are methane, propane in a gas grill, butane in a lighter, and octane in gasoline. Notice that these fuels all react with oxygen, which is necessary for anything to burn. In all combustion reactions, carbon dioxide, water, and energy are produced. When something burns, energy is released, which can be felt as heat and seen as light.
-
Q #7: A student notices a pattern of stripes on five tigers. Each of the five tigers has the same stripe pattern. Using his inductive reasoning, what does he logically assume based on this information?
A. The pattern continues to change over time.
B. Natural adaptations cause this pattern to occur
C. Each offspring will have the same stripe pattern
D. Ancestors of the tigers have different stripe patterns
Answer Explanation
Inductive reasoning involves making specific observations and using them to make broad statements. The student observes that all of the tigers have the same stripe pattern. He can use this observation to make the broad statement that all the tigers’ offspring will have the same stripe pattern.
Inductive reasoning involves drawing a general conclusion from specific observations. This form of reasoning is referred to as the “from the bottom up” approach. Information gathered from specific observations can be used to make a general conclusion about the topic under investigation. In other words, conclusions are based on observed patterns in data.
-
Q #8: What body system is the skeletal system most closely associated with when hematopoiesis happens?
A. Urinary system
B. Digestive system
C. Muscular system
D. Cardiovascular system
Answer Explanation
The cardiovascular system is closely associated with hematopoiesis because it includes the heart and blood vessels, which are responsible for circulating blood throughout the body. Hematopoiesis, the process of blood cell formation, primarily occurs in the bone marrow, which is part of the skeletal system. However, the cardiovascular system plays a crucial role in transporting these blood cells to various parts of the body once they are produced in the bone marrow.
So, while the skeletal system provides the site for hematopoiesis, the cardiovascular system is responsible for distributing the blood cells, making it the most closely associated system in this context.
-
Q #9: An intracellular chemical signal can be produced in the cell membrane. Once it is produced, where does it go?
A. To a different cell
B. To another part of the same cell
C. To a region right outside the cell
D. To an area with a high ion concentration
Answer Explanation
There are two major types of receptor molecules that respond to an intercellular chemical signal:
- Intracellular receptors: These receptors are located in either the cytoplasm or the nucleus of the cell. Signals diffuse across the cell membrane and bind to the receptor sites on intracellular receptors, of the same cell.
- Membrane-bound receptors: These receptors extend across the cell membrane, with their receptor sites on the outer surface of the cell membrane. They respond to intercellular chemical signals that are large, water-soluble molecules that do not diffuse across the cell membrane.
-
Q #10: What standard is used to make comparisons in experiments?
A. Sample size
B. Control group
C. Dependent variable
D. Independent variable
Answer Explanation
A control group is a factor that does not change during an experiment. Due to this, it is used as a standard for comparison with variables that do change such as a dependent variable.
Recall that these make up the scientific method, described below:
- Problem: The question created because of an observation. Example: Does the size of a plastic object affect how fast it naturally degrades in a lake?
- Research: Reliable information available about what is observed. Example: Learn how plastics are made and understand the properties of a lake.
- Hypothesis: A predicted solution to the question or problem. Example: If the plastic material is small, then it will degrade faster than a large particle.
- Experiment: A series of tests used to evaluate the hypothesis. Experiments consist of an independent variable that the researcher modifies and a dependent variable that changes due to the independent variable. They also include a control group used as a standard to make comparisons.
- Example: Collect plastic particles both onshore and offshore of the lake over time. Determine the size of the particles and describe the lake conditions during this time period.
- Observe: Analyze data collected during an experiment to observe patterns.
- Example: Analyze the differences between the numbers of particles collected in terms of size.
- Conclusion: State whether the hypothesis is rejected or accepted and summarize all results.
- Communicate: Report findings so others can replicate and verify the results.
Free Access on TEAS 7 Exams and Study Notes
- Access to all TEAS 7 Exams
- Performance Tracking and Analysis
- Well Documented and Explained Questions and Answers
- 2000+ Questions and Correct Answers: Answers Well Explained
- Libary of Detailed StudyNotes
- Topical Questions and Answers on Examinable topics
TEAS 7 Exams (Q&A)
TEAS 7 Study Notes
TEAS 7 Topical Tests

TEAS 7 Study Guides
Quick Links
Refer a Friend
Refer a friend and claim free unlimited access

© 2024 ExamGates Made with by ExamGates
