Which of the following molecules exhibits ionic bonding?
A. NaCl
B. CO2
C. C6H12O6
D. H2O
Sodium chloride exhibits lonic bonding due to the attraction between Na+ ions and Cl- ions.
Typically, elements on the opposite sides of the periodic table (a metal and a nonmetal) form ionic bonds. Carbon dioxide, water, and glucose exhibit covalent bonding. Typically, elements on the same side of the periodic table (two or more nonmetals) form covalent bonds.
Therefore, the Correct Answer is A.
More Questions on TEAS 7 Science
-
Q #1: In general, where in the periodic table of elements are the elements with the largest atomic radii located?
A. Upper-right corner
B. Upper-left corner
C. Bottom-left corner
D. Bottom-right comer
Answer Explanation
In general, atomic radius increases moving down a group due to the increasing number of electron shells. In general, atomic radius decreases moving from left to right across a period due to the increasing number of protons in the energy level. Therefore, atoms of elements in the bottom-left corner of the periodic table tend to have the largest atomic radii.
-
Q #2: What types of reaction is Cu (s) + 2AgNO3(aq) → 2Ag(s) + Cu(NO3)2(aq)?
A. Single replacement
B. Double replacement
C. Synthesis
D. Decomposition
Answer Explanation
This is a single replacement reaction in which copper replaces silver. The copper combines with the nitrate ions, and the silver precipitates out. Single replacement reactions have the general form of
A + BC →
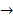 AC + B. Double replacement reactions have the general form of AB + CD→
AC + B. Double replacement reactions have the general form of AB + CD→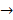 AD + CB. Synthesis reactions have the general form of A + B →
AD + CB. Synthesis reactions have the general form of A + B →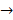 AB. Decomposition reactions have the general form AB→
AB. Decomposition reactions have the general form AB→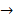 A+B.
A+B. -
Q #3: Which of the following are formed when the plasma membrane surrounds a particle outside of the cell?
A. Golgi bodies
B. Rough endoplasmic reticulum
C. Secretory vesicles
D. Endocytic vesicles
Answer Explanation
Endocytosis is a process by which cells absorb larger molecules or even tiny organisms, such as bacteria, that would not be able to pass through the plasma membrane. Endocytic vesicles containing molecules from the extracellular environment often undergo further processing once they enter the cell.
-
Q #4: Which structure of the nervous system carries action potential in the direction of a synapse?
A. Cell body
B. Axon
C. Neuron
D. Myelin
Answer Explanation
Axons carry action potential in the direction of synapses. Axons are the long, fiber-like structures that carry information from neurons. Electrical impulses travel along the body of the axons, some of which are up to a foot long.
A neuron is a type of cell that is responsible for sending information throughout the body. There are several types of neurons, including muscle neurons, which respond to instructions for movement; sensory neurons, which transmit information about the external world; and interneurons, which relay messages between neurons. Myelin is a fat that coats the nerves and ensures the accurate transmission of information in the nervous system.
-
Q #5: Which of the following are examples of homeostatic mechanisms?
A. Increasing heart rate when blood pressure is low, Consuming excess calories to gain weight
B. Lifting weights to increase muscle mass
C. Shivering when the body temperature falls
D. Shivering when the body temperature falls, Secreting insulin to decrease blood sugar concentration, Increasing heart rate when blood pressure is low
Answer Explanation
Homeostatic mechanisms are involuntary actions by organs, glands, tissues and cells to maintain balance within the body. For example, if a person becomes dehydrated, the kidneys will retain fluid by decreasing urine output.
Consuming excess calories and lifting weights are not involuntary actions, nor do they maintain the body in its baseline state. Rather, they are actions specifically taken to move the body away from its baseline.
-
Q #6: Given that there are over 100 million residential addresses in the United States, which of the following best describes a flaw of this survey as it is described?
A. There were not enough age groups to show a trend based on age.
B. The addresses were chosen randomly rather than strategically
C. The distribution of ages in the sample did not reflect the actual population
D. Each response represents only one person per address
Answer Explanation
While the random selection and large number of surveys sent out were a good start, to truly study the relationship between age and screen usage, it would have been better to have a few more age groups or just a direct age value from each participant rather than four large categories. As far as whether the age distribution accurately reflected the population, we don't have enough information to say if that was a problem or not. Also, the fact that each response is from just one person at the address is not a flaw but a reasonable way to get a good variety of responses
-
Q #7: Your class is competing with another class to determine who has the best plant color. Your class decides to test a couple of solutions to determine which would be best for overall plant color before competing. Starting with four sets of four plants, the class decides to water each set with a different solution. They water them once a week with 200ml of the following solutions: water, diet soda, 1% bleach solution, and a 1% salt solution. All plants are placed in the window that receives the recommended amount of light. After a month of testing, your class notices that only two plants are alive, but one of those two does not look healthy. Based on these results, which of the following would be a good hypothesis to design the next experiment around?
A. Plants need more than 200 mL of liquid per week.
B. Salt has no impact on plant health.
C. Salt contains useful nutrients for plants.
D. A combination of the solutions tested will produce healthier plants.
Answer Explanation
Since only 2 out of the initial 16 plants survived the whole month, it would be reasonable to test variables that were shared by all the plants. As such, the amount of liquid the plants need would be a good follow-up experiment. It is not specified as to which plants survived, so there is not enough information to think that testing the impact of salt is warranted. Additionally, since almost all the plants died, it is not reasonable to assume that a combination of the solutions would have an impact.
-
Q #8: Which is NOT a type of protein?
A. Fibrous
B. Membrane
C. Globular
D. Unsaturated
Answer Explanation
There are three types of proteins: fibrous, membrane, and globular. Fibrous or structural proteins consist of collagen, elastin, and keratin. Membrane proteins are interactive and anchored to a membrane. Globular proteins consist of functional proteins like enzymes, hemoglobin, and insulin.
Therefore unsaturated is the correct answer.
-
Q #9: How many different types of nucleotides are there in DNA?
A. One
B. Two
C. Four
D. Eight
Answer Explanation
There are four different nucleotides in DNA. Nucleotides are monomers of nucleic acids, composed of five- carbon sugars, a phosphate group, and a nitrogenous base. Nucleotides make up both DNA and RNA. They are essential for the recording of an organism's genetic information, which guides the actions of the various cells of the body.
-
Q #10: Which of the following answers shows CH4 +2O2=CO2 +H2O as balanced?
A. CH4 + 2O2 = 2CO2 + 2H2O
B. 2CH4 + 4O2 = 2CO2 + H2O
C. 2CH4 + O2 = 2CO2 + H2O
D. CH4 + 2O2 = CO2 + 2H2O
Answer Explanation
For the formula CH4 + 2O2 = CO2 + H2O to be balanced there must be an equal number of molecules on both the reactant and product sides. In this case, for the formula to be balanced, a coefficient of a 2 needs to be placed in front of the O2 and the H2O molecules. This would make both the reactant and product sides have one carbon, four hydrogens, and four oxygens
Free Access on TEAS 7 Exams and Study Notes
- Access to all TEAS 7 Exams
- Performance Tracking and Analysis
- Well Documented and Explained Questions and Answers
- 2000+ Questions and Correct Answers: Answers Well Explained
- Libary of Detailed StudyNotes
- Topical Questions and Answers on Examinable topics
TEAS 7 Exams (Q&A)
TEAS 7 Study Notes
TEAS 7 Topical Tests

TEAS 7 Study Guides
Quick Links
Refer a Friend
Refer a friend and claim free unlimited access

© 2024 ExamGates Made with by ExamGates
