Which sequence describes the hierarchy level of biological organization?
A. Kingdom, phylum, class, order, family, genus, and species
B. Genus, class, kingdom, species, order, phylum, and family
C. Genus, class, kingdom, species, order, phylum, and family
D. Species, kingdom, genus, class, family, phylum, and order
Taxonomy is the process of classifying, describing, and naming organisms. There are seven levels in the Linnaean taxonomic system, starting with the broadest level, kingdom, and ending with the species level. For example, in the image the genus level contains two types of bears, but the species level shows one type. Additionally, organisms in each level are found in the level above it. For example, organisms in the order level are part of the class level. This classification system is based on physical similarities across living things. It does not account for molecular or genetic similarities.
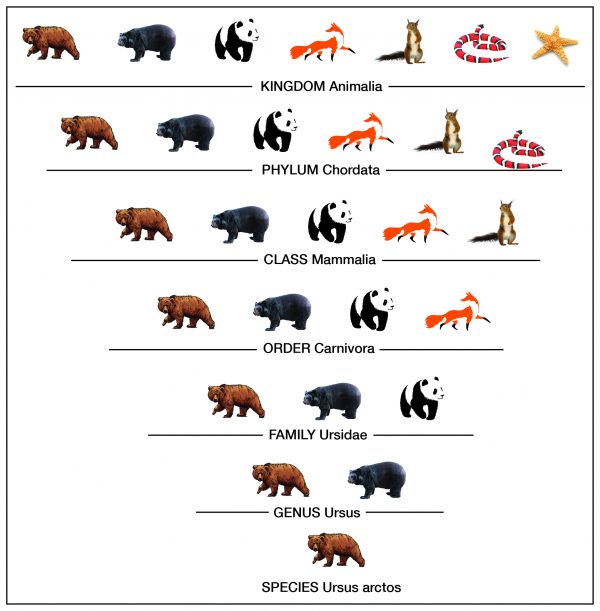
Therefore, the Correct Answer is A.
More Questions on TEAS 7 Science
-
Q #1: What is the correct order of the stages of the cell cycle?
A. G1,S,G2,M
B. G2,S,G1,M
C. M,S,G2,G1
D. S,M,G1,G1
Answer Explanation
The cell cycle is an organized process divided into two phases: interphase and the M (mitotic) phase. During interphase, the cell grows and copies its DNA. After the cell reaches the M phase, division of the two new cells can occur. The G1, S, and G2 phases make up interphase.
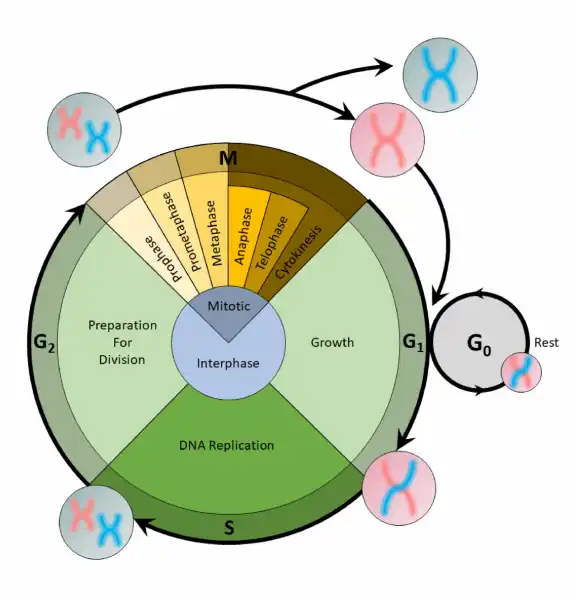
- G1: The first gap phase, during which the cell prepares to copy its DNA
- S: The synthesis phase, during which DNA is copied
- G2 : The second gap phase, during which the cell prepares for cell division
It may appear that little is happening in the cell during the gap phases. Most of the activity occurs at the level of enzymes and macromolecules. The cell produces things like nucleotides for synthesizing new DNA strands, enzymes for copying the DNA, and tubulin proteins for building the mitotic spindle. During the S phase, the DNA in the cell doubles, but few other signs are obvious under the microscope. All the dramatic events that can be seen under a microscope occur during the M phase: the chromosomes move, and the cell splits into two new cells with identical nuclei.
-
Q #2: In which state of matter are the intermolecular forces between particles in a substance the strongest?
A. Gas
B. Liquid
C. Plasma
D. Solid
Answer Explanation
In solids, particles are usually closer together than in other states of matter because of the strong cohesive forces between the particles.
- Solids, liquids, gases, and plasmas differ from one another in the amount of energy that the particles have and the strength of the cohesive forces that hold the particles together.
- Cohesion is the tendency of particles of the same kind to stick to each other.
- A solid has the lowest amount of energy because its particles are packed close together. Liquids have more energy than a solid, and gases have more energy than solids or liquids because the cohesive forces are very weak.
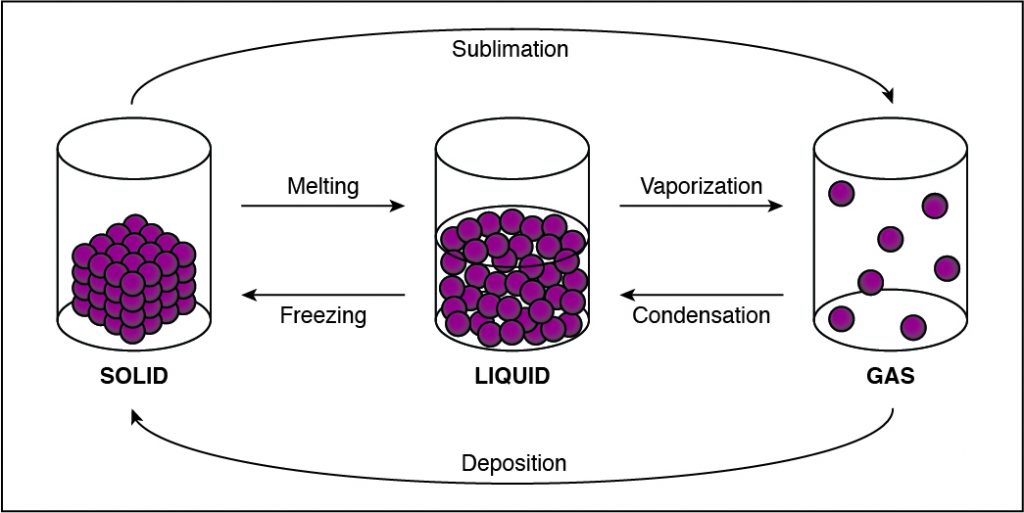
-
Q #3: Which of the following atoms is a cation?
A. 14 protons, 14 neutrons, 18 electrons
B. 34 protons, 45 neutrons, 36 electrons
C. 35 protons, 44 neutrons, 35 electrons
D. 82 protons, 125 neutrons, 78 electrons
Answer Explanation
Because it has more protons than electrons, this atom has a positive charge and can be classified as a cation. When a metal such as sodium reacts to become stable, it loses its valence electrons. At first, it is a neutral atom with 11 protons and 11 electrons. When it loses an electron, the number of protons does not change, and the atom has 11 protons and 10 electrons. Because there is one more positively charged proton, a cation forms. A cation is an ion with a net positive charge.
-
Q #4: An intracellular chemical signal can be produced in the cell membrane. Once it is produced, where does it go?
A. To a different cell
B. To another part of the same cell
C. To a region right outside the cell
D. To an area with a high ion concentration
Answer Explanation
There are two major types of receptor molecules that respond to an intercellular chemical signal:
- Intracellular receptors: These receptors are located in either the cytoplasm or the nucleus of the cell. Signals diffuse across the cell membrane and bind to the receptor sites on intracellular receptors, of the same cell.
- Membrane-bound receptors: These receptors extend across the cell membrane, with their receptor sites on the outer surface of the cell membrane. They respond to intercellular chemical signals that are large, water-soluble molecules that do not diffuse across the cell membrane.
-
Q #5: Why did it take many years for the cell theory to be developed?
A. Advancements in microscopy took place slowly.
B. Cells were difficult to isolate for experimental analysis
C. Researchers believed a cell formed from preexisting cells
D. Scientists already proved that cells were essential for life.
Answer Explanation
Robert Hooke discovered the first cells in the mid-eighteenth century. The cell theory is a theory because it is supported by a significant number of experimental findings. The cell theory took many years to be developed because microscopes were not powerful enough to make such observations.
This theory, or in-depth explanation, about cells consists of three parts:
- All living things are composed of one or more cells.
- Cells are alive and represent the basic unit of life.
- All cells are produced from pre-existing cells.
-
Q #6: What phase is the cell cycle part of?
A. Interphase
B. Metaphase
C. Prophase
D. Telophase
Answer Explanation
Before mitosis or meiosis occurs, interphase must happen. This is when the cell cycle takes place. The cell cycle is an organized process divided into two phases: interphase and the M (mitotic) phase. During interphase, the cell grows and copies its DNA. After the cell reaches the M phase, division of the two new cells can occur. The G1, S, and G2 phases make up interphase.
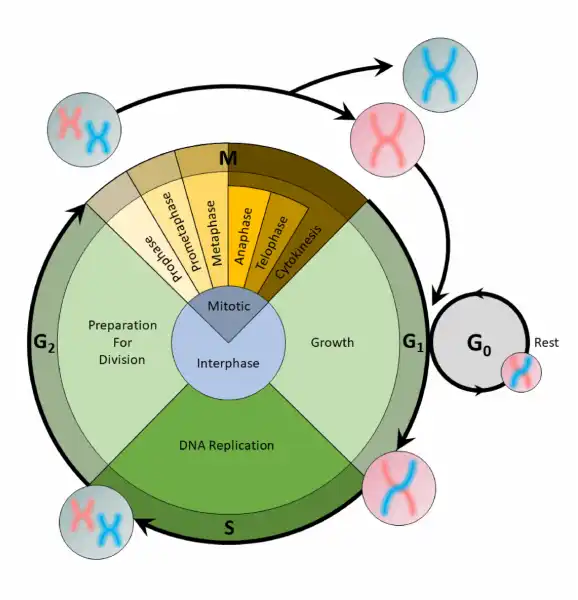
-
Q #7: Which example is part of the scientific method?
A. A student reads about a new way to harness energy from the sun.
B. A researcher studies the effects of car exhaust on how people breathe.
C. A researcher analyzes how many plants respond well to a new fertilizer
D. A student discovers how insulin plays a role in the development of diabetes
Answer Explanation
One step of the scientific method is to analyze information or data collected from the experiment to conclude whether the hypothesis is supported.
Recall that these make up the scientific method, described below:
- Problem: The question created because of an observation. Example: Does the size of a plastic object affect how fast it naturally degrades in a lake?
- Research: Reliable information available about what is observed. Example: Learn how plastics are made and understand the properties of a lake.
- Hypothesis: A predicted solution to the question or problem. Example: If the plastic material is small, then it will degrade faster than a large particle.
- Experiment: A series of tests used to evaluate the hypothesis. Experiments consist of an independent variable that the researcher modifies and a dependent variable that changes due to the independent variable. They also include a control group used as a standard to make comparisons.
- Example: Collect plastic particles both onshore and offshore of the lake over time. Determine the size of the particles and describe the lake conditions during this time period.
- Observe: Analyze data collected during an experiment to observe patterns.
- Example: Analyze the differences between the numbers of particles collected in terms of size.
- Conclusion: State whether the hypothesis is rejected or accepted and summarize all results.
- Communicate: Report findings so others can replicate and verify the results.
-
Q #8: In the following single-replacement reaction, ______ replaces ______. Cl2+2NaI→2NaCl+I2
A. sodium, iodine
B. chlorine, iodine
C. chlorine, sodium
D. sodium, chlorine
Answer Explanation
In this reaction, chlorine (Cl2) is an element in the reaction that replaces iodine in the compound sodium iodide (NaI). This allows chlorine to form a compound with sodium (NaCl) and leaves iodine (I2) as an element.
Synthesis reactions involve two or more reactants (A and B) combining to form one product (AB). In the example provided, hydrogen (H2) and oxygen (O2) begin as separate elements. At the end of the reaction, the hydrogen and oxygen atoms are bonded in a molecule of water (H2O).
Decomposition reactions have only one reactant (AB) that breaks apart into two or more products (A and B). In the example above, hydrogen peroxide (H2O2) breaks apart into two smaller molecules: water (H2O) and oxygen (O2).
Single-replacement reactions involve two reactants, one compound (AB) and one element (C). In this type of reaction, one element replaces another to form a new compound (AC), leaving one element by itself (B). In the example, zinc replaces hydrogen in hydrochloric acid (HCl). As a result, zinc forms a compound with chlorine, zinc chloride (ZnCl2), and hydrogen (H2) is left by itself.
Double-replacement reactions involve two reactants, both of which are compounds made of two components (AB and CD). In the example, silver nitrate, composed of silver (Ag1+) and nitrate (NO31-) ions, reacts with sodium chloride, composed of sodium (Na1+) and chloride (Cl1-) ions. The nitrate and chloride ions switch places to produce two compounds that are different from those in the reactants.
Combustion reactions occur when fuels burn, and they involve specific reactants and products, as seen in the examples below. Some form of fuel that contains carbon and hydrogen is required. Examples of such fuels are methane, propane in a gas grill, butane in a lighter, and octane in gasoline. Notice that these fuels all react with oxygen, which is necessary for anything to burn. In all combustion reactions, carbon dioxide, water, and energy are produced. When something burns, energy is released, which can be felt as heat and seen as light.
-
Q #9: What raw inorganic material would an autotroph most likely use to create chemical energy for growth?
A. carbon dioxide
B. minerals in soil
C. decaying matter
D. sugar molecules
Answer Explanation
Autotrophs are organisms that use basic raw materials in nature, like the sun, to make energy-rich biomolecules. Minerals are naturally inorganic.
Autotrophs are organisms that make energy-rich biomolecules from raw material in nature. They do this by using basic energy sources such the sun. This explains why most autotrophs rely on photosynthesis to transform sunlight into usable food that can produce energy necessary for life. Plants and certain species of bacteria are autotrophs.
-
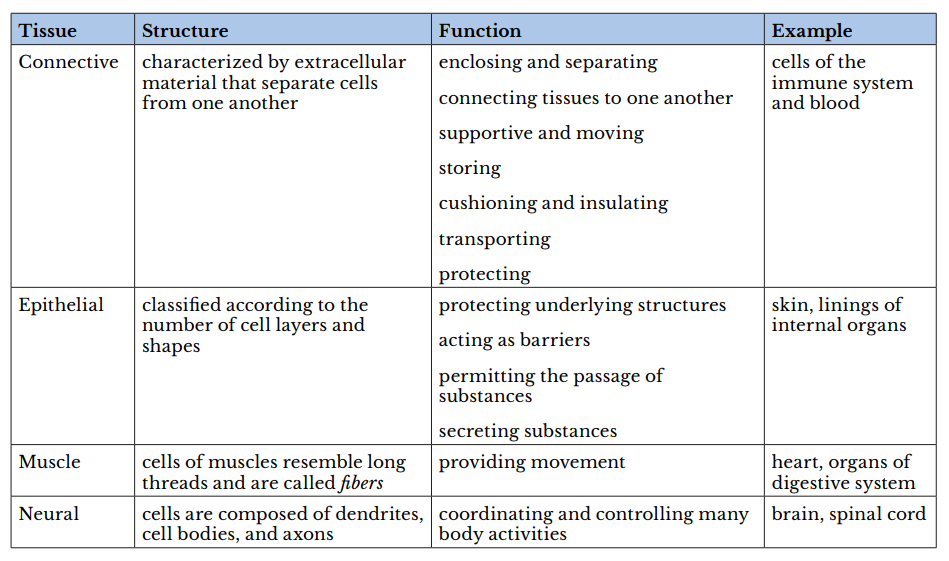
Q #10: Which of the following types of tissues include cells of the immune system and of the blood?
A. Connective
B. Epithelial
C. Muscle
D. Neural
Answer Explanation
A tissue is a group of cells with similar structure and function and similar extracellular substances located between the cells. The table below describes the four primary tissues found in the human body.
body.
Free Access on TEAS 7 Exams and Study Notes
- Access to all TEAS 7 Exams
- Performance Tracking and Analysis
- Well Documented and Explained Questions and Answers
- 2000+ Questions and Correct Answers: Answers Well Explained
- Libary of Detailed StudyNotes
- Topical Questions and Answers on Examinable topics
TEAS 7 Exams (Q&A)
TEAS 7 Study Notes
TEAS 7 Topical Tests

TEAS 7 Study Guides
Quick Links
Refer a Friend
Refer a friend and claim free unlimited access

© 2024 ExamGates Made with by ExamGates
