Which type of bond is responsible for the unique properties of water and plays a crucial role in the structure of DNA and proteins?
A. Hydrogen bonds.
B. Covalent bonds.
C. Ionic bonds.
D. Van der Waals forces.
The correct answer is choice A. Hydrogen bonds.
Hydrogen bonds are responsible for the unique properties of water and play a crucial role in the structure of DNA and proteins.
Hydrogen bonds are weak electrostatic attractions between a hydrogen atom covalently bonded to an electronegative atom and another electronegative atom.
Choice B.
Covalent bonds is incorrect because covalent bonds are strong chemical bonds formed by the sharing of electrons between two atoms.
Choice C.
Ionic bonds is incorrect because ionic bonds are chemical bonds formed by the transfer of electrons from one atom to another, resulting in the formation of ions.
Choice D.
Van der Waals forces is incorrect because Van der Waals forces are weak intermolecular forces that arise from temporary dipoles induced in atoms or molecules.
Therefore, the Correct Answer is A.
More Questions on TEAS 7 Science
-
Q #1: What is a control group used for in scientific studies?
A. To establish causality by isolating the effect of an independent variable.
B. To establish the effect of a dependent variable on an independent variable.
C. To control the impact of extraneous variables on the dependent variable.
D. To control the impact of extraneous variables on the independent variable.
Answer Explanation
A control group is used in scientific studies to establish causality by isolating the effect of an independent variable.
The control group serves as a baseline or comparison group that does not receive the treatment or intervention being tested.
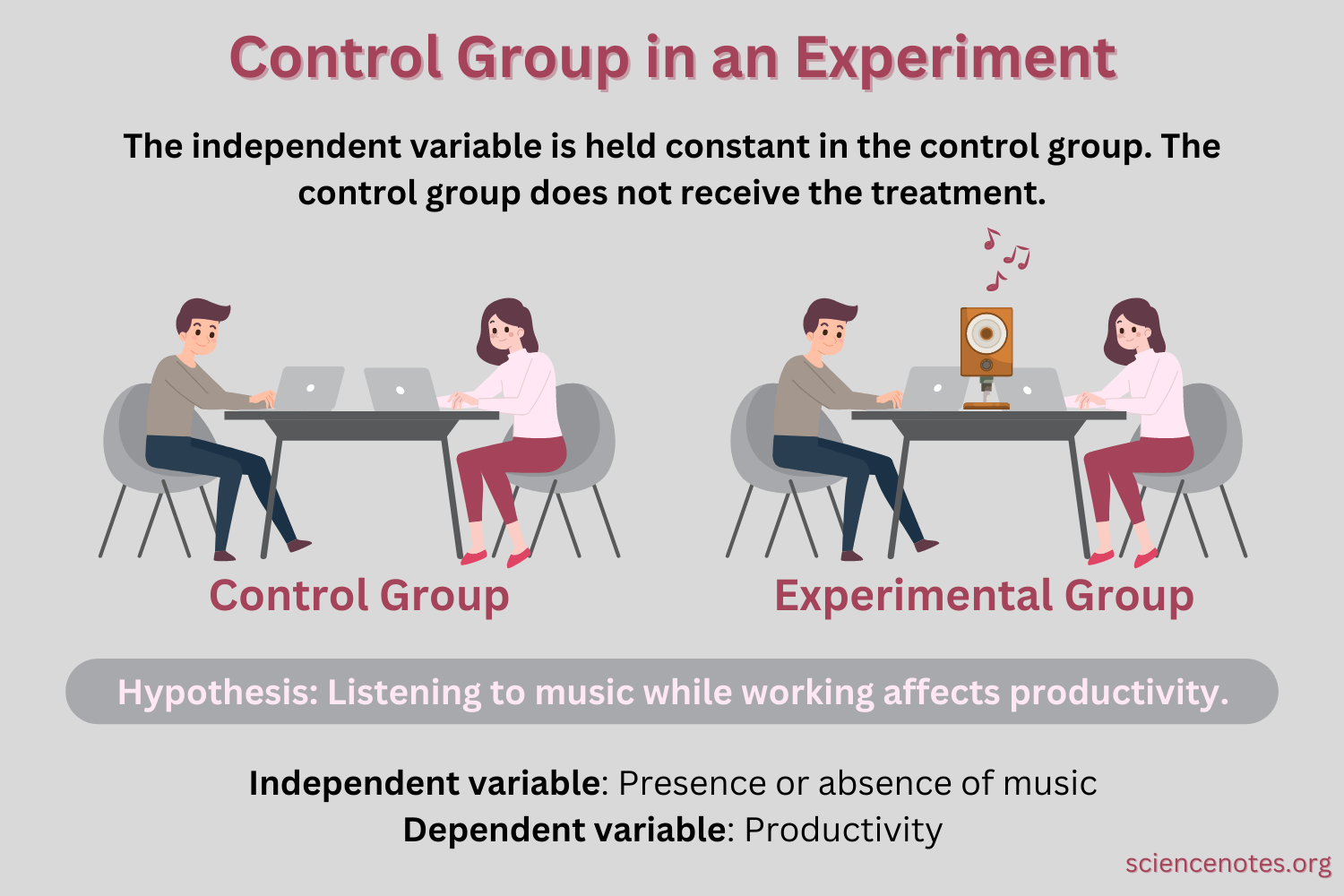
By comparing the results of the control group to the experimental group, researchers can determine if any observed changes are due to the independent variable or if they are due to chance or other factors.
Choice B is incorrect because a control group is not used to establish the effect of a dependent variable on an independent variable.
Choice C is incorrect because while a control group can help control for the impact of extraneous variables on the dependent variable, its primary purpose is to isolate the effect of the independent variable.
Choice D is incorrect because a control group is not used to control for the impact of extraneous variables on the independent variable.
-
Q #2: How does the use of a catalyst affect the activation energy of a chemical reaction?
A. It increases the activation energy required for the reaction.
B. It decreases the activation energy required for the reaction.
C. It has no effect on the activation energy required for the reaction.
D. It increases the rate of reaction but has no effect on the activation energy.
Answer Explanation
The correct answer is choice B.
It decreases the activation energy required for the reaction.
A catalyst provides a new reaction pathway in which a lower activation energy is offered.
This allows more reactant molecules to collide with enough energy to surmount the smaller energy barrier, increasing the rate of reaction 2.
Choice A, It increases the activation energy required for the reaction, is not the correct answer because it describes the opposite effect of a catalyst.
Choice C, It has no effect on the activation energy required for the reaction, is not the correct answer because a catalyst does have an effect on activation energy.
Choice D, It increases the rate of reaction but has no effect on the activation energy, is not the correct answer because a catalyst increases the rate of reaction by decreasing the activation energy.
-
Q #3: Which subatomic particle contributes to the positive charge of an atom?
A. Proton
B. Neutron
C. Electron
D. Nucleon
Answer Explanation
Protons contribute to the positive charge of an atom.
Protons are subatomic particles with a positive charge found in the nucleus of an atom.
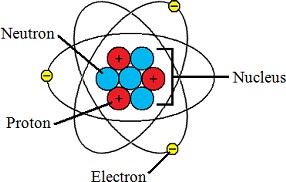
Choice B is incorrect because neutrons are neutral and do not have a charge. Choice C is incorrect because electrons have a negative charge.
Choice D is incorrect because nucleons refer to both protons and neutrons, but only protons contribute to the positive charge of an atom.
-
Q #4: What is the name of the process in which an atom loses or gains electrons to form an ion?
A. Ionization
B. Oxidation
C. Reduction
D. Isotopic decay
Answer Explanation
Ionization is the process in which an atom loses or gains electrons to form an ion.
An ion is an atom or molecule that has a net electrical charge due to the loss or gain of one or more electrons.
Choice B is not the best answer because oxidation refers to the loss of electrons from an atom or molecule.
Choice C is not the best answer because reduction refers to the gain of electrons by an atom or molecule.
Choice D is not the best answer because isotopic decay refers to the process in which an unstable atomic nucleus loses energy by emitting radiation
-
Q #5: Which type of bond is responsible for the unique properties of water and plays a crucial role in the structure of DNA and proteins?
A. Hydrogen bonds.
B. Covalent bonds.
C. Ionic bonds.
D. Van der Waals forces.
Answer Explanation
The correct answer is choice A. Hydrogen bonds.
Hydrogen bonds are responsible for the unique properties of water and play a crucial role in the structure of DNA and proteins.
Hydrogen bonds are weak electrostatic attractions between a hydrogen atom covalently bonded to an electronegative atom and another electronegative atom.
Choice B.
Covalent bonds is incorrect because covalent bonds are strong chemical bonds formed by the sharing of electrons between two atoms.
Choice C.
Ionic bonds is incorrect because ionic bonds are chemical bonds formed by the transfer of electrons from one atom to another, resulting in the formation of ions.
Choice D.
Van der Waals forces is incorrect because Van der Waals forces are weak intermolecular forces that arise from temporary dipoles induced in atoms or molecules.
-
Q #6: Which of the following is an example of a storage form of glucose in the human body?
A. Starch
B. Glycogen
C. Fructose
D. Cellulose
Answer Explanation
Glycogen is the storage form of glucose in the human body.
It is a polysaccharide that is stored primarily in the liver and muscle tissue and can be broken down into glucose when the body needs energy.
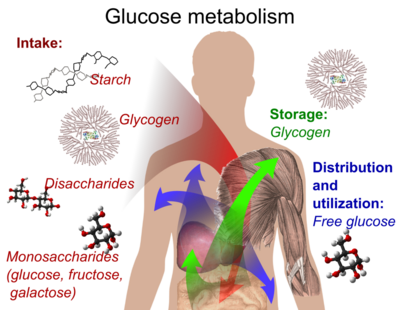
Choice A is incorrect because starch is a storage form of glucose in plants, not in the human body.
Choice C is incorrect because fructose is a simple sugar, not a storage form of glucose.
Choice D is incorrect because cellulose is a structural carbohydrate found in plant cell walls, not a storage form of glucose in the human body.
-
Q #7: What is a primer in DNA sequencing?
A. A short piece of double-stranded DNA that binds to the template DNA and acts as a "starter" for the polymerase.
B. A short piece of double-stranded DNA that binds to the primer and acts as a "starter" for the template.
C. A short piece of single-stranded DNA that binds to the template DNA and acts as a "starter" for the polymerase.
D. A short piece of single-stranded DNA that binds to the polymerase and acts as a "starter" for the template.
Answer Explanation
A primer is a short single-stranded DNA fragment used in certain laboratory techniques, such as the polymerase chain reaction (PCR).
In the PCR method, a pair of primers hybridizes with the sample DNA and defines the region that will be amplified.
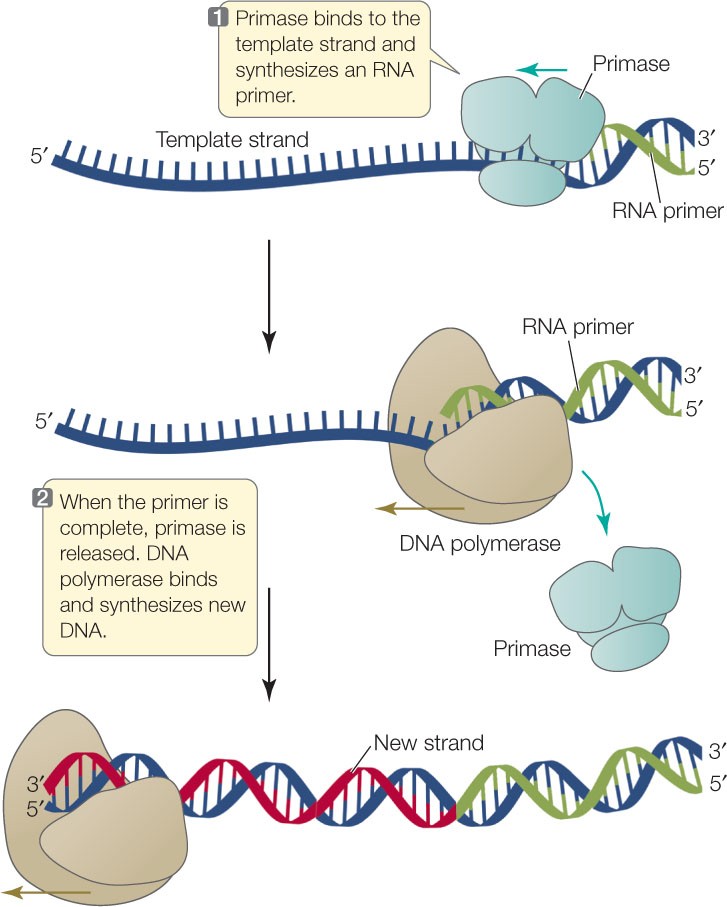
Choice A) A short piece of double-stranded DNA that binds to the template DNA and acts as a “starter” for the polymerase is incorrect because primers are single-stranded, not double-stranded.
Choice B) A short piece of double-stranded DNA that binds to the primer and acts as a “starter” for the template is incorrect because it does not make sense for a primer to bind to itself.
Choice D) A short piece of single-stranded DNA that binds to the polymerase and acts as a “starter” for the template is incorrect because primers bind to the template DNA, not to the polymerase.
Note: DNA primers are used instead of RNA primers in DNA sequencing and PCR because DNA is more stable, specific, and compatible with the enzymes and processes involved in these techniques.
-
Q #8: A nurse is reviewing the results of a patient’s DNA sequencing test, which was performed to diagnose a genetic disorder. The nurse notices that the patient has a mutation in one of the bases of the DNA. Which of the following is the correct term for this type of mutation?
A. Deletion
B. Insertion
C. Substitution
D. Inversion
Answer Explanation
The correct answer is choice C. Substitution.
A substitution mutation is a type of point mutation where one base in the DNA sequence is replaced by another base.
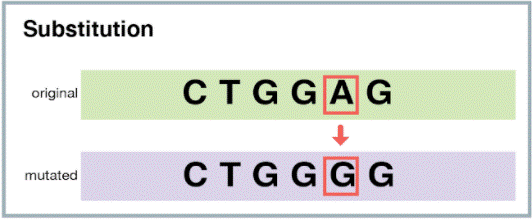
Choice A is incorrect because a deletion mutation occurs when one or more bases are removed from the DNA sequence.
Choice B is incorrect because an insertion mutation occurs when one or more bases are added to the DNA sequence.
Choice D is incorrect because an inversion mutation occurs when a segment of DNA is reversed within the chromosome.
-
Q #9: What is the hallmark of adaptive immunity?
A. Rapid recruitment of immune cells to sites of infection and inflammation
B. Antigen-independent defense mechanism
C. Immunologic memory
D. Non-specific host-defense mechanisms .
Answer Explanation
Immunologic memory is the hallmark of adaptive immunity.
Immunologic memory enables the host to mount a more rapid and efficient immune response upon subsequent exposure to the antigen.
Choice A is incorrect because rapid recruitment of immune cells to sites of infection and inflammation is a characteristic of innate immunity.
Choice B is incorrect because antigen-independent defense mechanisms are characteristic of innate immunity.
Choice D is incorrect because non-specific host-defense mechanisms are characteristic of innate immunity.
-
Q #10: A patient with chronic renal failure is undergoing hemodialysis. What process allows for the removal of waste products and excess fluid from the patient's bloodstream during hemodialysis?
A. Active transport.
B. Osmosis
C. Diffusion
D. Facilitated diffusion.
Answer Explanation
Diffusion.
During hemodialysis, waste products and excess fluids are removed from the blood by diffusion 1.
Diffusion is a separation process in which particles that are dissolved in a solution are relocated from an area of higher concentration in the blood to an area of lower concentration in the dialysate.
Choice A.
Active transport is incorrect because active transport is a process that uses energy to move molecules against a concentration gradient.
Choice B.
Osmosis is incorrect because osmosis is the movement of water molecules across a semipermeable membrane from an area of higher water concentration to an area of lower water concentration.
Choice D.
Facilitated diffusion is incorrect because facilitated diffusion is a process where molecules move down their concentration gradient with the help of carrier proteins.
Free Access on TEAS 7 Exams and Study Notes
- Access to all TEAS 7 Exams
- Performance Tracking and Analysis
- Well Documented and Explained Questions and Answers
- 2000+ Questions and Correct Answers: Answers Well Explained
- Libary of Detailed StudyNotes
- Topical Questions and Answers on Examinable topics
TEAS 7 Exams (Q&A)
TEAS 7 Study Notes
TEAS 7 Topical Tests

TEAS 7 Study Guides
Quick Links
Refer a Friend
Refer a friend and claim free unlimited access

© 2024 ExamGates Made with by ExamGates
