Properties of Acids
Properties of Acids
Acids exhibit several characteristic properties that distinguish them from other substances. Understanding these properties is essential for identifying acids and predicting their behavior in various chemical reactions.
A. Sour Taste:
- Acids typically possess a sour taste. However, it's important to note that tasting acids is highly discouraged due to their corrosive nature and potential health hazards.
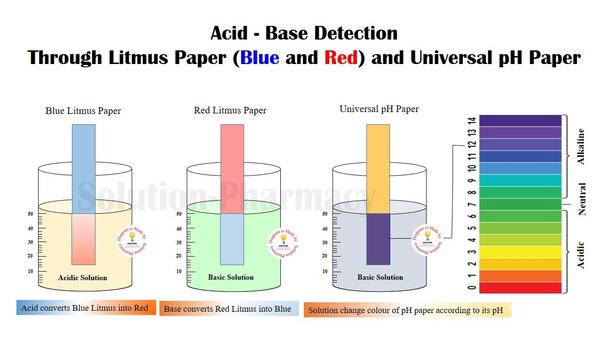
B. Effect on Indicators:
- Acids change the color of certain indicators. For example, they turn blue litmus paper red and may cause phenolphthalein to remain colorless.
C. Reaction with Metals:
- Acids react with certain metals to produce hydrogen gas and salts. The general reaction can be represented as follows:
Acid + Metal => Salt + Hydrogen gas
- For instance, hydrochloric acid (HCl) reacts with zinc (Zn) to produce zinc chloride (ZnCl2) and hydrogen gas (H2):
2HCl + Zn => ZnCl2 + H2
D. Reaction with Bases:
- Acids neutralize bases through a chemical reaction known as neutralization. This reaction results in the formation of water and a salt. For example, the neutralization of hydrochloric acid (HCl) with sodium hydroxide (NaOH) produces sodium chloride (NaCl) and water (H2O):
HCl + NaOH => NaCl + H2O
E. Conductivity:
- Acids conduct electricity when dissolved in water due to the presence of ions. Strong acids ionize completely in solution, leading to higher conductivity compared to weak acids, which only partially ionize.
F. pH Scale and Acidity:
- Acids have pH values below 7 on the pH scale, indicating their acidic nature. The lower the pH value, the stronger the acid. Strong acids have pH values close to 0, while weak acids have pH values closer to 7.
Understanding these properties of acids is crucial for their identification, characterization, and prediction of their behavior in various chemical reactions and applications.