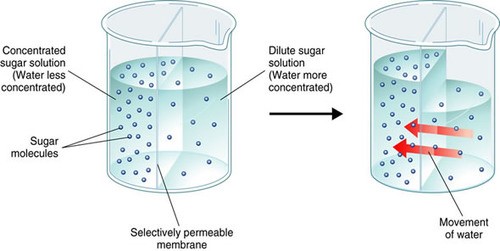
In a hypertonic solution, water flows through aquaporins embedded in the plasma membrane of the cell. This type of transport is best known as which of the following?
A. Facilitated diffusion
B. Active transport
C. Osmosis
D. Diffusion
Osmosis is the movement of water molecules across a selectively permeable membrane from an area of higher water concentration to an area of lower water concentration.
In a hypertonic solution, the concentration of solutes outside the cell is higher than inside the cell, so water flows out of the cell through aquaporins embedded in the plasma membrane to balance the concentration gradient.
Choice A.
Facilitated diffusion is not correct because it is a type of passive transport that involves the movement of molecules across a membrane through specific transport proteins, but it does not specifically refer to the movement of water molecules.
Choice B.
Active transport is not correct because it is a type of transport that involves the movement of molecules against their concentration gradient and requires energy in the form of ATP, but osmosis is a passive process that does not require energy.
Choice D.
Diffusion is not correct because it refers to the movement of molecules from an area of higher concentration to an area of lower concentration, but it does not specifically refer to the movement of water molecules.
Therefore, the Correct Answer is C.