The triple point of a substance is the temperature and pressure at which the substance exists as which of the following?
A. Simultaneously in sol, gel, and plasma phases
B. As a gel with solid and liquid trapped in gas
C. As a sol with gas and solid trapped in liquid
D. Simultaneously in solid, liquid, and gas phases
The triple point of a substance is the temperature and pressure at which the substance exists simultaneously in solid, liquid, and gas phases ¹. In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.
The other options are not correct because they do not accurately describe the triple point of a substance. Sol, gel, and plasma are not phases that coexist at the triple.
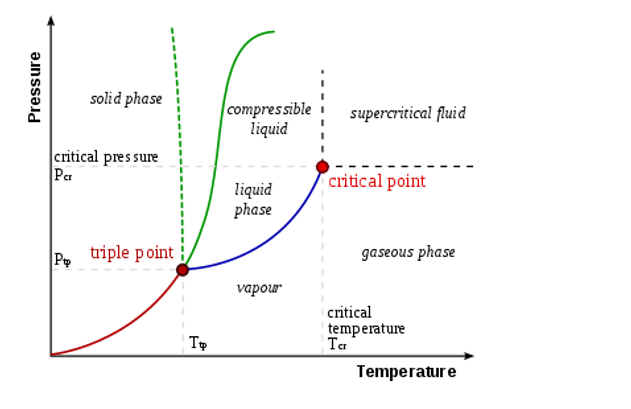
Therefore, the Correct Answer is D.