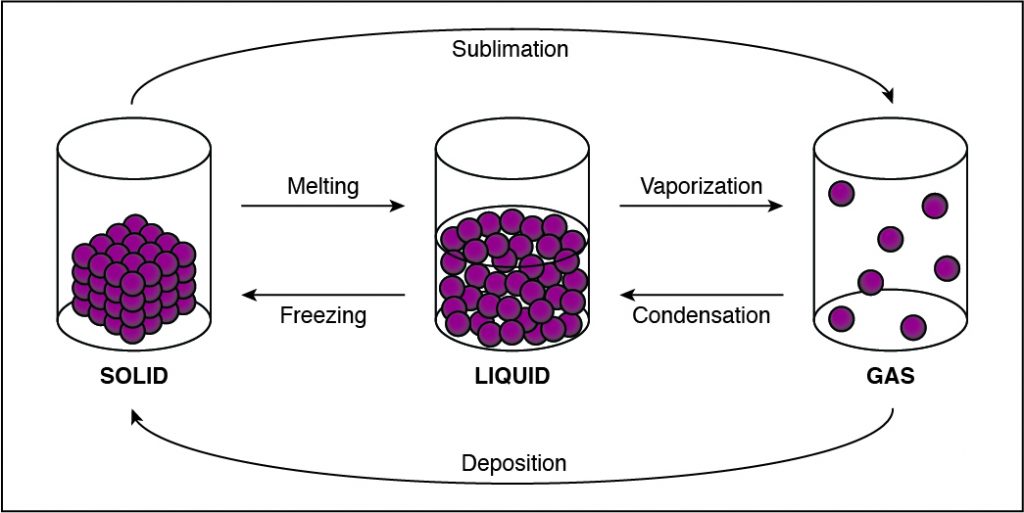
What type of reaction is described by the following equation? ZnBr2(aq) + 2KOH(aq) → Zn(OH)2(s) + 2KBr(aq)
A. Synthesis
B. Decomposition
C. Single-Replacement
D. Double-Replacement
In this reaction, two elements are trading places hence double-replacement. In the reactants, zinc and bromide ions are together, and potassium and hydroxide ions are together. In the products, zinc and hydroxide ions are together, and potassium and bromide ions are together.
Therefore, the Correct Answer is D.