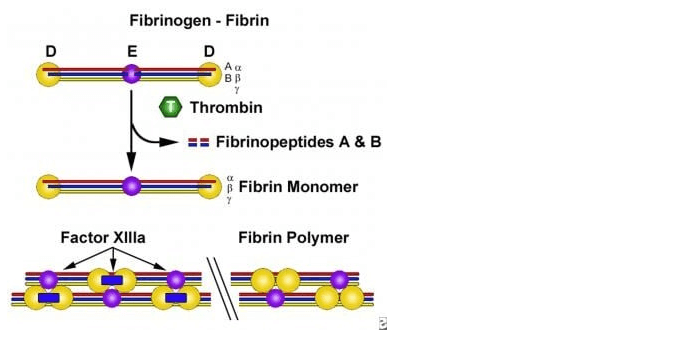
Which of the following properties does soap, an emulsifier, have that make it useful for washing dirt off one’s hands with water?
A. Soap’s dual polar and nonpolar nature helps bond oil and water
B. Soap’s acidity causes grime to precipitate into the water
C. Soap’s enzymatic action helps to dissolve grime into small particles
D. Soap’s rough texture physically scours grime off surfaces
The correct answer is a. Soap’s dual polar and nonpolar nature helps bond oil and water. Soap is an emulsifier, which means that it has both polar and nonpolar regions. The polar regions of soap molecules are atracted to water, while the nonpolar regions are atracted to oil and grease. This allows soap to bond with both water and oil, helping to remove dirt and grime from surfaces.
B. Soap’s acidity does not cause grime to precipitate into the water.
C. Soap does not have enzymatic action that helps to dissolve grime into small particles.
D. Soap’s texture does not physically scour grime off surfaces.
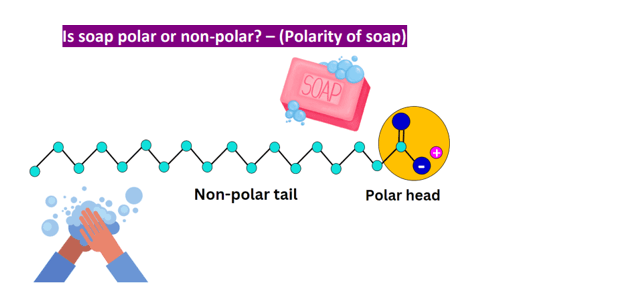
Therefore, the Correct Answer is A.